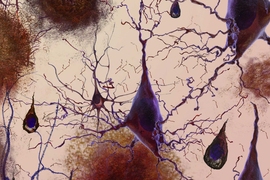
People with a gene variant called APOE4 have a higher risk of developing late-onset Alzheimer’s disease: APOE4 is three times more common among Alzheimer’s patients than it is among the general population. However, little is known about why this version of the APOE gene, which is normally involved in metabolism and transport of fatty molecules such as cholesterol, confers higher risk for Alzheimer’s.
To shed light on this question, MIT neuroscientists have performed a comprehensive study of APOE4 and the more common form of the gene, APOE3. Studying brain cells derived from a type of induced human stem cells, the researchers found that APOE4 promotes the accumulation of the beta amyloid proteins that cause the characteristic plaques seen in the brains of Alzheimer’s patients.
“APOE4 influences every cell type that we studied, to facilitate the development of Alzheimer’s pathology, especially amyloid accumulation,” says Li-Huei Tsai, director of MIT’s Picower Institute for Learning and Memory and the senior author of the study.
The researchers also found that they could eliminate the signs of Alzheimer’s in brain cells with APOE4 by editing the gene to turn it into the APOE3 variant.
Picower Institute Research Scientist Yuan-Ta Lin and former postdoc Jinsoo Seo are the lead authors of the paper, which appears in the May 31 online edition of Neuron.
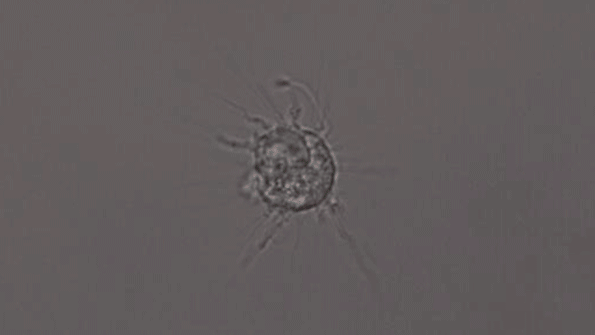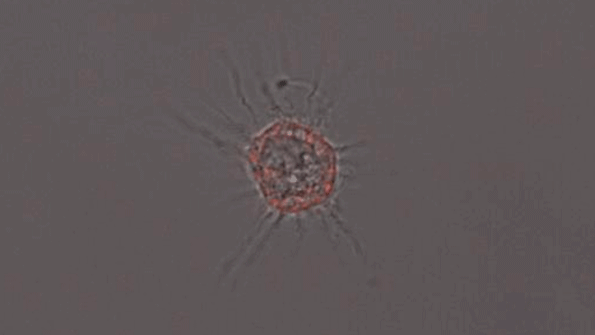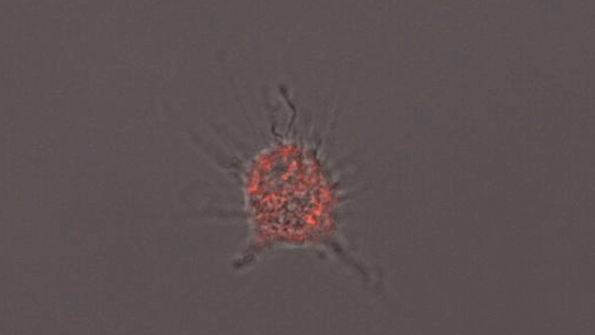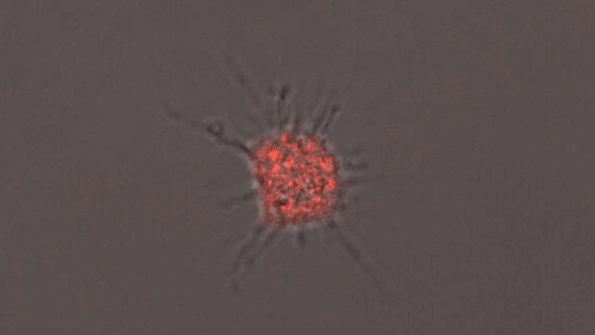
A microglia-like cell grown from human cells expressing the APOE4 protein. (Courtesy of the researchers)
Amyloid accumulation
APOE, also called apolipoprotein E, comes in three variants, known as 2, 3, and 4. APOE binds to cholesterol and lipids in cells’ environments, enabling the cells to absorb the lipids. In the brain, cells known as astrocytes produce lipids, which are then secreted and taken up by neurons with the help of APOE.
Among the general population, about 8 percent of people have APOE2, 78 percent have APOE3, and 14 percent have APOE4. However, among people with late onset, nonfamilial Alzheimer’s, which accounts for 95 percent of all cases, the profile is very different: Only 4 percent have APOE2, and the percentage with APOE3 drops to 60 percent. APOE4 shows a dramatic increase: Thirty-seven percent of late-onset Alzheimer’s patients carry this version of the gene.
“APOE4 is by far the most significant risk gene for late-onset, sporadic Alzheimer’s disease,” Tsai says. “However, despite that, there really has not been a whole lot of research done on it. We still don’t have a very good idea of why APOE4 increases the disease risk.”
Previous studies have shown that people with the APOE4 gene have higher levels of amyloid proteins, but little is known about why that is.
In this study, the MIT team set out to answer that question using human induced pluripotent stem cells — stem cells derived from skin or other cell types. They were able to stimulate those stem cells to differentiate into three different types of brain cells: neurons, astrocytes, and microglia.
Using the gene-editing system CRISPR/Cas9, the researchers genetically converted APOE3 in stem cells derived from a healthy subject to APOE4. Because the cells were genetically identical except for the APOE gene, any differences seen between them could be attributed to that gene.
In neurons, the researchers found that cells expressing APOE3 and APOE4 differed in the expression of hundreds of genes — about 250 genes went down and 190 went up in cells with APOE4. In astrocytes, the numbers were even higher, and they were highest of all in microglia: In APOE4 microglia, more than 1,100 genes showed reduced activity, while 300 became more active.
These genetic changes also translated to differences in cell behavior. Neurons with APOE4 formed more synapses, and they secreted higher levels of amyloid protein.
In APOE4 astrocytes, the researchers found that cholesterol metabolism was highly dysregulated. The cells produced twice as much cholesterol as APOE3 astrocytes, and their ability to remove amyloid proteins from their surroundings was dramatically impaired.
Microglia were similarly affected. These cells, whose normal function is to help remove foreign matter, including amyloid proteins and pathogens such as bacteria, became much slower at this task when they had the APOE4 gene.
The researchers also found that they could reverse most of these effects by using CRISPR/Cas9 to convert the APOE4 gene to APOE3 in brain cells derived from induced stem cells from a patient with late-onset Alzheimer’s disease.
Disrupting cell behavior
In another experiment, the researchers created three-dimensional “organoids,” or miniature brains, from cells with genes that are known to cause early-onset Alzheimer’s. These organoids had high levels of amyloid aggregates, but when they were exposed to APOE3 microglia, most of the aggregates were cleared away. In contrast, APOE4 microglia did not efficiently clear the aggregates.
Tsai said she believes that APOE4 may disrupt specific signaling pathways within brain cells, leading to the changes in behavior that the researchers saw in this study.
“From this gene expression profiling, we can narrow down to certain signaling pathways that are dysregulated by APOE4,” she says. “I think that this definitely can reveal potential targets for therapeutic intervention.”
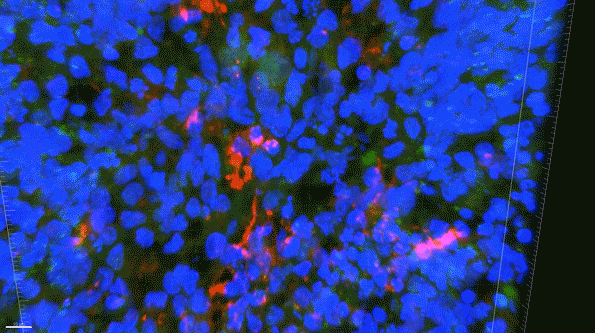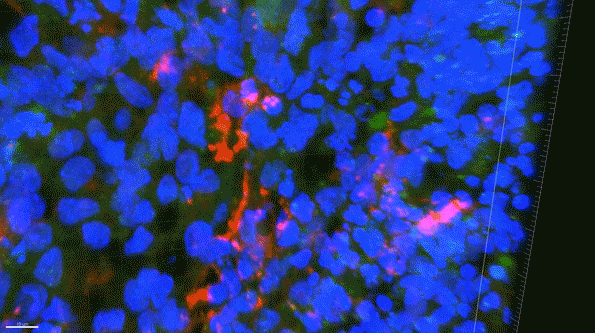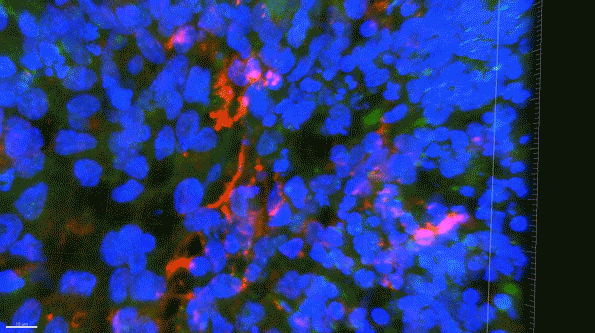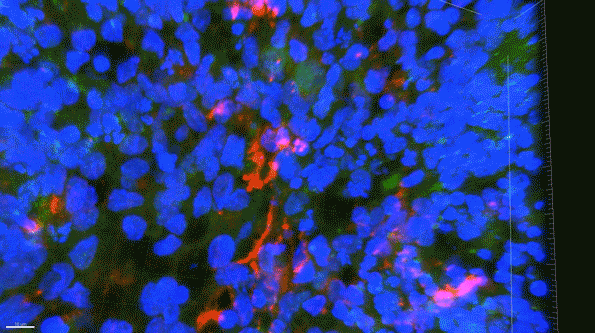
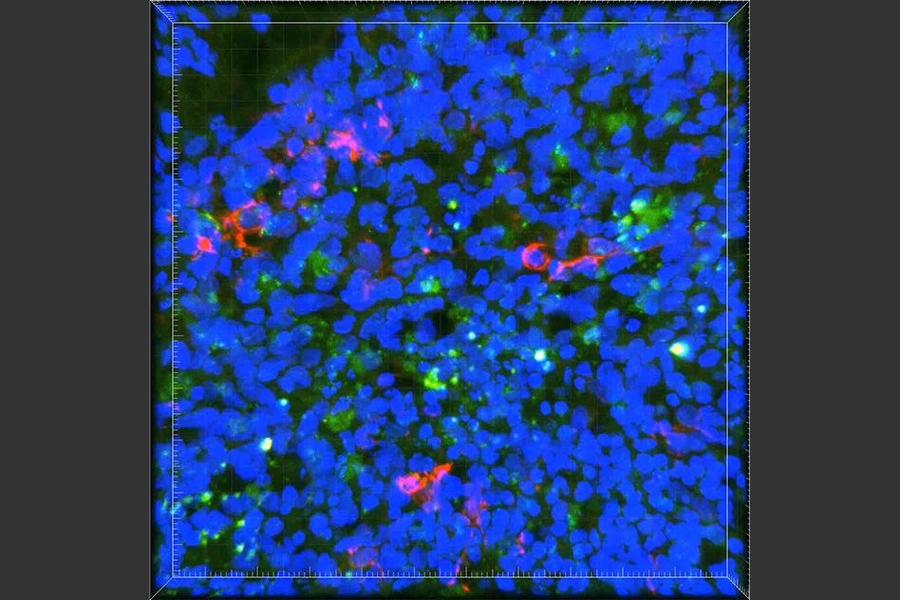
In this 3D brain “organoid,” microglia-like cells, labeled in red, fail to properly clear amyloid proteins (green) from the brain tissue. (Courtesy of the researchers)
The findings also suggest that if gene-editing technology could be made to work in humans, which many biotechnology companies are now trying to achieve, it could offer a way to treat Alzheimer’s patients who carry the APOE4 gene.
“If you can convert the gene from E4 to E3, a lot of the Alzheimer’s associated characteristics can be diminished,” Tsai says.
Li Gan, a professor of neurology at the University of California at San Francisco, says that the findings significantly add to scientists’ knowledge of APOE’s role in Alzheimer’s disease, particularly since most previous studies of the gene were done in mice.
“The importance of APOE cannot be overstated,” says Gan, who was not involved in the research. “This study is very exciting because APOE4 is the most predominant risk factor for Alzheimer’s disease, and this is the first study to systematically compare E3 and E4 in human cells.”
The research was funded by the National Research Foundation of Korea, the National Institutes of Health, the Glenn Foundation for Medical Research, the Robert A. and Renee E. Belfer Family Foundation, and Cure Alzheimer’s Fund.
![“…[I]f humans behave similarly to mice in response to this treatment, I would say the potential is just enormous, because it’s so noninvasive, and it’s so accessible,” says Li-Huei Tsai, the Picower Professor of Neuroscience, when describing a new treatment for Alzheimer’s disease.](/sites/default/files/styles/news_article__archive/public/images/201612/MIT-li-huei-tsai.jpg?itok=jrC2K2AI)