Audio
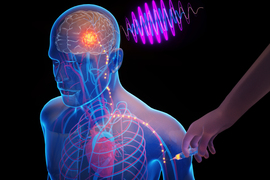
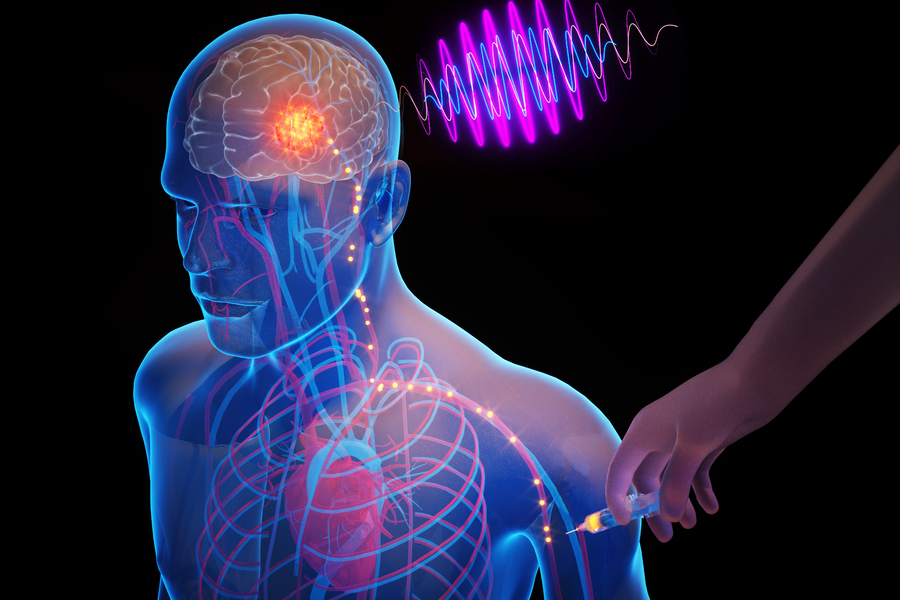
What if clinicians could place tiny electronic chips in the brain that electrically stimulate a precise target, through a simple injection in the arm? This may someday help treat deadly or debilitating brain diseases, while eliminating surgery-related risks and costs.
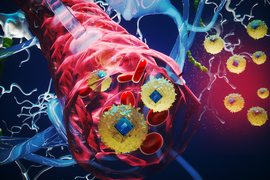
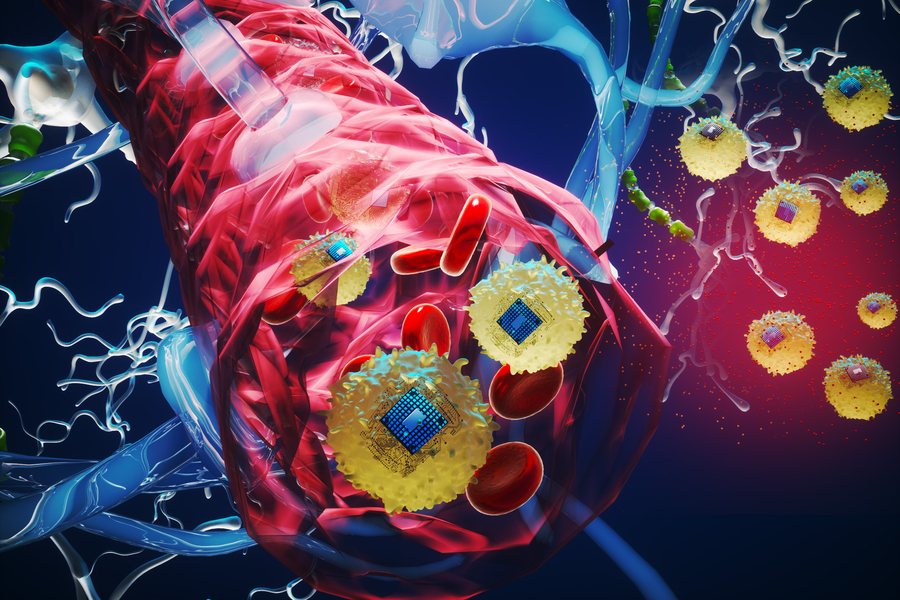
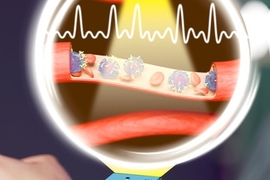
MIT researchers have taken a major step toward making this scenario a reality. They developed microscopic, wireless bioelectronics that could travel through the body’s circulatory system and autonomously self-implant in a target region of the brain, where they would provide focused treatment.
In a study on mice, the researchers show that after injection, these miniscule implants can identify and travel to a specific brain region without the need for human guidance. Once there, they can be wirelessly powered to provide electrical stimulation to the precise area. Such stimulation, known as neuromodulation, has shown promise as a way to treat brain tumors and diseases like Alzheimer’s and multiple sclerosis.
Moreover, because the electronic devices are integrated with living, biological cells before being injected, they are not attacked by the body’s immune system and can cross the blood-brain barrier while leaving it intact. This maintains the barrier’s crucial protection of the brain.
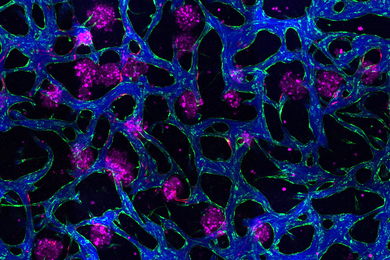
The researchers demonstrated the use of this technology, which they call “circulatronics,” to target brain inflammation, a major factor in the progression of many neurological diseases. They show that the implants can provide localized neuromodulation deep inside the brain achieving high precision, to within several microns around the target area.
In addition, the biocompatible implants do not damage surrounding neurons.
While brain implants usually require hundreds of thousands of dollars in medical costs and risky surgical procedures, circulatronics technology holds the potential to make therapeutic brain implants accessible to all by eliminating the need for surgery, says Deblina Sarkar, the AT&T Career Development Associate Professor in the MIT Media Lab and MIT Center for Neurobiological Engineering, head of the Nano-Cybernetic Biotrek Lab, and senior author of a study on the work.
She is joined on the paper by lead author Shubham Yadav, an MIT graduate student; as well as others at MIT, Wellesley College, and Harvard University. The research appears today in Nature Biotechnology.
Hybrid implants
The team has been working on circulatronics for more than six years. The electronic devices, each about one-billionth the length of a grain of rice, are composed of organic semiconducting polymer layers sandwiched between metallic layers to create an electronic heterostructure.
They are fabricated using CMOS-compatible processes in the MIT.nano facilities, and then integrated with living cells to create cell-electronics hybrids. To do this, the researchers lift the devices off the silicon wafer on which they are fabricated, so they are free-floating in a solution.
“The electronics worked perfectly when they were attached to the substrate, but when we originally lifted them off, they didn’t work anymore. Solving that challenge took us more than a year,” Sarkar says.
Key to their operation is the high wireless power conversion efficiency of the tiny electronics. This enables the devices to work deep inside the brain and still harness enough energy for neuromodulation.
The researchers use a chemical reaction to bond the electronic devices to cells. In the new study, they fused the electronics with a type of immune cell called monocytes, which target areas of inflammation in the body. They also applied a fluorescent dye, allowing them to trace the devices as they crossed the intact blood-brain barrier and self-implanted in the target brain region.
While they explored brain inflammation in this study, the researchers hope to use different cell types and engineer the cells to target specific regions of the brain.
“Our cell-electronics hybrid fuses the versatility of electronics with the biological transport and biochemical sensing prowess of living cells,” Sarkar says. “The living cells camouflage the electronics so that they aren’t attacked by the body’s immune system and they can travel seamlessly through the bloodstream. This also enables them to squeeze through the intact blood-brain barrier without the need to invasively open it.”
Over the course of about four years, the team tried many methods to autonomously and noninvasively cross the blood-brain barrier before they perfected this cellular integration technique.
In addition, because the circulatronics devices are so tiny, they offer much higher precision than conventional electrodes. They can self-implant, leading to millions of microscopic stimulation sites that take the exact shape of the target region.
Their small size also enables the biocompatible devices to live alongside neurons without causing harmful effects. Through a series of biocompatibility tests, the researchers found that circulatronics can safely integrate among neurons without impacting the brain processes behind cognition or motion.
After the devices have self-implanted in the target region, a clinician or researcher uses an external transmitter to provide electromagnetic waves, in the form of near-infrared light, that power the technology and enable electrical stimulation of the neurons.
Targeting deadly diseases
The Sarkar lab is currently working on developing their technology to treat multiple diseases including brain cancer, Alzheimer’s disease, and chronic pain.
The tiny size and self-implantation capabilities of circulatronics devices could make them well-suited to treat brain cancers such as glioblastoma that cause tumors at multiple locations, some of which may be too small to identify with imaging techniques. They may also provide new avenues for treating especially deadly cancers like diffuse intrinsic pontine glioma, an aggressive type of tumor found in the brain stem that usually cannot be surgically removed.
“This is a platform technology and may be employed to treat multiple brain diseases and mental illnesses,” Sarkar says. “Also, this technology is not just confined to the brain but could also be extended to other parts of the body in future.”
The researchers hope to move the technology into clinical trials within three years through the recently launched startup Cahira Technologies.
They are also exploring integration of additional nanoelectronic circuits into their devices to enable functionalities including sensing, feedback based on-chip data analysis, and capabilities such as creating synthetic electronic neurons.
“Our tiny electronic devices seamlessly integrate with the neurons and co-live and co-exist with the brain cells creating a unique brain-computer symbiosis. We are working dedicatedly to employ this technology for treating neural diseases, where drugs or standard therapies fail, for alleviating human suffering and envision a future where humans could transcend beyond diseases and biological limitations,” says Sarkar.