Cancer drugs that stimulate the body’s immune system to attack tumors are a promising way to treat many types of cancer. However, some of these drugs produce too much systemic inflammation when delivered intravenously, making them harmful to use in patients.
MIT researchers have now come up with a possible way to get around that obstacle. In a new study, they showed that when immunostimulatory prodrugs — inactive drugs that require activation in the body — are tuned for optimal activation timing, the drugs provoke the immune system to attack tumors without the side effects that occur when the active form of the drug is given.
The researchers designed prodrugs with bottlebrush-like structures based on a class of compounds called imidazoquinolines (IMDs). Mice treated with these bottlebrush prodrugs designed with optimized activation kinetics showed a significant reduction in tumor growth, with no side effects. The researchers hope that this approach could be used to boost immune system responses in cancer patients, especially when combined with other immunotherapy drugs or cancer vaccines.
“Our bottlebrush prodrug library enabled us to show an immunological effect of controlling immunotherapy kinetics, allowing us to boost immune responses while minimizing the side effects,” says Sachin Bhagchandani, an MIT graduate student who is the lead author of the study. “This kind of approach opens up avenues for scientists who want to decouple toxicity from some promising immunotherapy agents.”
Jeremiah Johnson, an MIT professor of chemistry, and Darrell Irvine, the Underwood-Prescott Professor with appointments in MIT’s departments of Biological Engineering and of Materials Science and Engineering, are the senior authors of the paper, which appears today in Science Advances. Irvine is also an associate director of MIT’s Koch Institute for Integrative Cancer Research and a member of the Ragon Institute of MGH, MIT, and Harvard.
Tailored prodrugs
Organic molecules known as IMDs bind to cell receptors called Toll-like receptors that are found on macrophages and other cells of the innate immune system. When activated, these cells begin producing cytokines and other inflammatory molecules.
In 1997, the FDA approved topical IMD drugs to treat certain types of skin cancer. Since then, many other IMD drugs have been tested in clinical trials for a variety of types of cancer, but none of these were approved, in part because the drugs produced too much systemic inflammation.
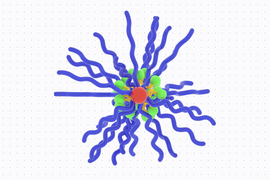
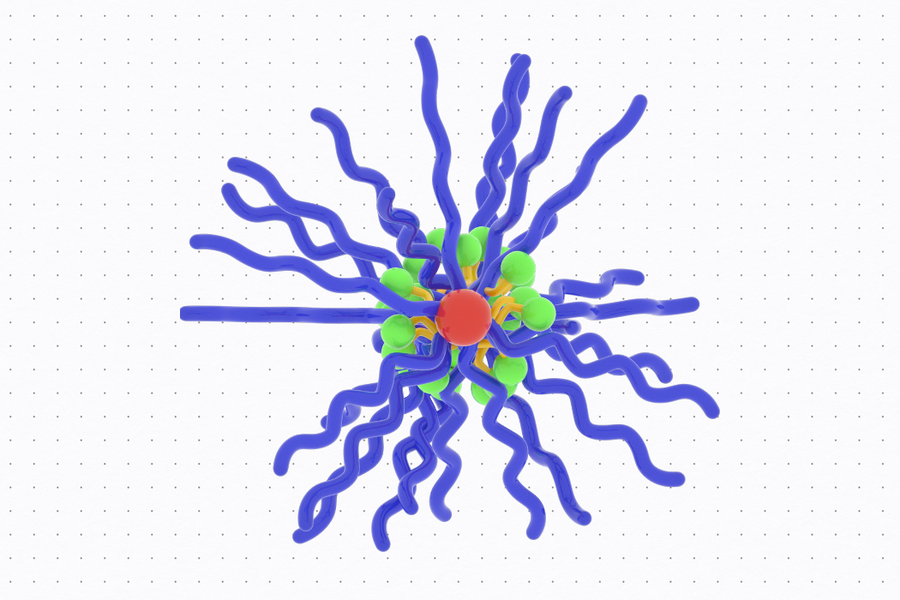
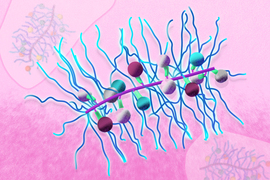
The MIT team set out to explore whether prodrugs of IMDs, which are inactivated until turned “on” in the tumor microenvironment, could reduce those side effects. In recent years, Johnson’s lab has developed a novel type of prodrug platform shaped like a bottlebrush. These nanoscale, cylindrical structures consist of chains that extend from a central backbone, giving the molecule a bottlebrush-like structure. Inactivated drugs are bound along the bottlebrush backbone through cleavable linkers that define the rate of active IMD release.
The researchers generated and compared six bottlebrush prodrugs that only differed by their release rate, in order to investigate how prodrug activation kinetics impact antitumor responses. Using these bottlebrush prodrugs, the researchers hoped they could deliver active IMDs to tumors while avoiding release into the bloodstream.
“Our ability to synthesize six bottlebrush prodrugs with identical sizes and shapes uniquely allows us to isolate and study release kinetics as a key variable. Excitingly, we find that it is possible to identify prodrug structures that limit IMD exposure to the whole body, thereby avoiding toxicity, and that activate in tumors to give antitumor efficacy,” Johnson says.
In preliminary studies in cells and mice, the researchers found that the fastest-activating prodrugs did cause immune-related side effects, including weight loss and elevated cytokine levels. However, the medium- and slow-releasing versions did not produce these effects.
The researchers then tested the IMD bottlebrush prodrugs in two different mouse models of colon cancer. Because the prodrugs are so small (approximately10 nanometers), they are able to efficiently accumulate in tumors. Once there, they get taken up by innate immune cells, where their linkers are cleaved. The resulting release of active IMDs causes immune cells to release cytokines and other molecules that create a pro-inflammatory environment. This series of events activates nearby T cells to attack the tumor.
In both models, mice treated with the bottlebrush prodrugs showed significantly slowed tumor growth. When the treatment was combined with a checkpoint blockade inhibitor — another class of immunotherapy drug — tumors were completely eliminated in about 20 percent of the mice.
While mice treated with the IMD used in this study, known as resiquimod, showed weight loss, elevated cytokine levels, and reduction in white blood cell count, as expected, mice given resiquimod bottlebrush prodrugs did not show any of these effects.
“Our molecules were able to safely reduce these effects by controlling how much of the active drug is released in the blood,” Bhagchandani says. “If you minimize release of the active compound there, then you’re able to get anti-tumor effects at the tumor site without the systemic side effects.”
Enhanced response
The findings suggest that the most promising use for IMD bottlebrush prodrugs could be to give them along with another drug that stimulates the immune response. Another possibility is using IMD bottlebrush prodrugs as adjuvants to enhance the immune system’s response to cancer vaccines.
“The ability of the bottlebrush prodrug strategy to change both where the drug accumulates in the body and when it is active is very attractive for activating immune responses against cancer or other disease safely,” Irvine says.
This research was funded by the Marble Center for Cancer Nanomedicine; the Ragon Institute of MGH, MIT and Harvard; the Koch Institute Frontier Research Program via the Curt and Kathy Marble Cancer Research Fund; a graduate fellowship from the Ludwig Center at the Koch Institute; and the National Cancer Institute.
Other authors of the paper include Farrukh Vohidov, Lauren Milling, Evelyn Yuzhou Tong, Christopher Brown, Michelle Ramseier, Bin Liu, Timothy Fessenden, Hung Nguyen, Gavin Kiel, Lori Won, Robert Langer, Stefani Spranger, and Alex Shalek.