A team of researchers led by Feng Zhang at the McGovern Institute for Brain Research at MIT and the Broad Institute of MIT and Harvard has uncovered the first programmable RNA-guided system in eukaryotes — organisms that include fungi, plants, and animals.
In a study published today in Nature, the team describes how the system is based on a protein called Fanzor. They showed that Fanzor proteins use RNA as a guide to target DNA precisely, and that Fanzors can be reprogrammed to edit the genome of human cells. The compact Fanzor systems have the potential to be more easily delivered to cells and tissues as therapeutics than CRISPR-Cas systems, and further refinements to improve their targeting efficiency could make them a valuable new technology for human genome editing.
CRISPR-Cas was first discovered in prokaryotes (bacteria and other single-cell organisms that lack nuclei) and scientists including those in Zhang’s lab have long wondered whether similar systems exist in eukaryotes. The new study demonstrates that RNA-guided DNA-cutting mechanisms are present across all kingdoms of life.
“CRISPR-based systems are widely used and powerful because they can be easily reprogrammed to target different sites in the genome,” says Zhang, senior author on the study, the James and Patricia Poitras Professor of Neuroscience in the MIT departments of Biological Engineering and Brain and Cognitive Sciences, an investigator at MIT’s McGovern Institute, a core institute member at the Broad Institute, and a Howard Hughes Medical Institute investigator. “This new system is another way to make precise changes in human cells, complementing the genome editing tools we already have.”
Searching the domains of life
A major aim of the Zhang lab is to develop genetic medicines using systems that can modulate human cells by targeting specific genes and processes. “A number of years ago, we started to ask, ‘What is there beyond CRISPR, and are there other RNA-programmable systems out there in nature?’” says Zhang.
Two years ago, Zhang lab members discovered a class of RNA-programmable systems in prokaryotes called OMEGAs, which are often linked with transposable elements, or “jumping genes,” in bacterial genomes and likely gave rise to CRISPR-Cas systems. That work also highlighted similarities between prokaryotic OMEGA systems and Fanzor proteins in eukaryotes, suggesting that the Fanzor enzymes might also use an RNA-guided mechanism to target and cut DNA.
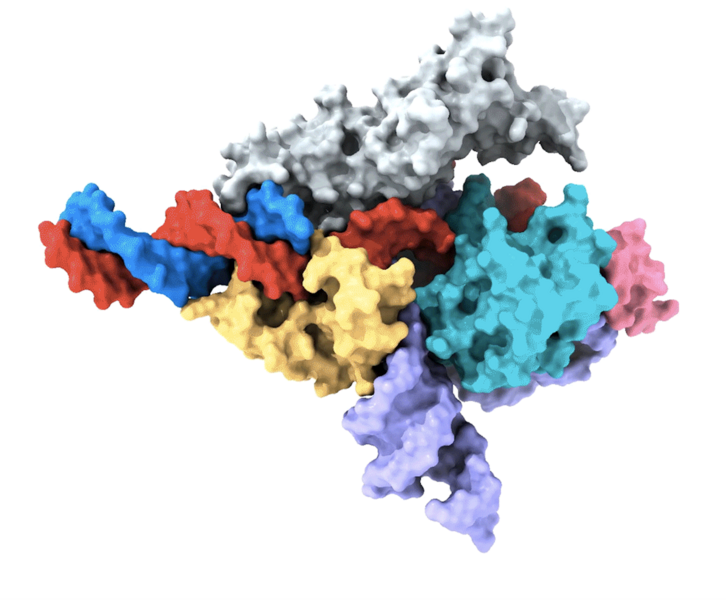
In the new study, the researchers continued their work on RNA-guided systems by isolating Fanzors from fungi, algae, and amoeba species, in addition to a clam known as the northern quahog. Co-first author Makoto Saito of the Zhang lab led the biochemical characterization of the Fanzor proteins, showing that they are DNA-cutting endonuclease enzymes that use nearby non-coding RNAs known as ωRNAs to target particular sites in the genome. It is the first time this mechanism has been found in eukaryotes, such as animals.
Unlike CRISPR proteins, Fanzor enzymes are encoded in the eukaryotic genome within transposable elements, and the team’s phylogenetic analysis suggests that the Fanzor genes have migrated from bacteria to eukaryotes through so-called horizontal gene transfer.
“These OMEGA systems are more ancestral to CRISPR and they are among the most abundant proteins on the planet, so it makes sense that they have been able to hop back and forth between prokaryotes and eukaryotes,” says Saito.
No collateral damage
To explore Fanzor’s potential as a genome editing tool, the researchers demonstrated that it can generate insertions and deletions at targeted genome sites within human cells. The researchers found the Fanzor system to initially be less efficient at snipping DNA than CRISPR-Cas systems, but by systematic engineering, they introduced a combination of mutations into the protein that increased its activity 10-fold. Additionally, unlike some CRISPR systems and the OMEGA protein TnpB, the team found that a fungal-derived Fanzor protein did not exhibit “collateral activity,” where an RNA-guided enzyme cleaves its DNA target as well as degrading nearby DNA or RNA. The results suggest that Fanzors could potentially be developed as efficient genome editors.
Co-first author Peiyu Xu led an effort to analyze the molecular structure of the Fanzor/ωRNA complex and illustrate how it latches onto DNA to cut it. Fanzor shares structural similarities with its prokaryotic counterpart CRISPR-Cas12 protein, but the interaction between the ωRNA and the catalytic domains of Fanzor is more extensive, suggesting that the ωRNA might play a role in the catalytic reactions. “We are excited about these structural insights for helping us further engineer and optimize Fanzor for improved efficiency and precision as a genome editor,” said Xu.
Like CRISPR-based systems, the Fanzor system can be easily reprogrammed to target specific genome sites, and Zhang said it could one day be developed into a powerful new genome editing technology for research and therapeutic applications. The abundance of RNA-guided endonucleases like Fanzors further expands the number of OMEGA systems known across kingdoms of life and suggests that there are more yet to be found.
“Nature is amazing. There’s so much diversity,” says Zhang. “There are probably more RNA-programmable systems out there, and we’re continuing to explore and will hopefully discover more.”
The paper’s other authors include Guilhem Faure, Samantha Maguire, Soumya Kannan, Han Altae-Tran, Sam Vo, AnAn Desimone, and Rhiannon Macrae.
Support for this work was provided by the Howard Hughes Medical Institute; Poitras Center for Psychiatric Disorders Research at MIT; K. Lisa Yang and Hock E. Tan Molecular Therapeutics Center at MIT; Broad Institute Programmable Therapeutics Gift Donors; The Pershing Square Foundation, William Ackman, and Neri Oxman; James and Patricia Poitras; BT Charitable Foundation; Asness Family Foundation; Kenneth C. Griffin; the Phillips family; David Cheng; Robert Metcalfe; and Hugo Shong.