Audio
To help mitigate climate change, companies are using bioreactors to grow algae and other microorganisms that are hundreds of times more efficient at absorbing CO2 than trees. Meanwhile, in the pharmaceutical industry, cell culture is used to manufacture biologic drugs and other advanced treatments, including lifesaving gene and cell therapies.
Both processes are hampered by cells’ tendency to stick to surfaces, which leads to a huge amount of waste and downtime for cleaning. A similar problem slows down biofuel production, interferes with biosensors and implants, and makes the food and beverage industry less efficient.
Now, MIT researchers have developed an approach for detaching cells from surfaces on demand, using electrochemically generated bubbles. In an open-access paper published in Science Advances, the researchers demonstrated their approach in a lab prototype and showed it could work across a range of cells and surfaces without harming the cells.
“We wanted to develop a technology that could be high-throughput and plug-and-play, and that would allow cells to attach and detach on demand to improve the workflow in these industrial processes,” says Professor Kripa Varanasi, senior author of the study. “This is a fundamental issue with cells, and we’ve solved it with a process that can scale. It lends itself to many different applications.”
Joining Varanasi on the study are co-first authors Bert Vandereydt, a PhD student in mechanical engineering, and former postdoc Baptiste Blanc.
Solving a sticky problem
The researchers began with a mission.
“We’ve been working on figuring out how we can efficiently capture CO2 across different sources and convert it into valuable products for various end markets,” Varanasi says. “That’s where this photobioreactor and cell detachment comes into the picture.”
Photobioreactors are used to grow carbon-absorbing algae cells by creating tightly controlled environments involving water and sunlight. They feature long, winding tubes with clear surfaces to let in the light algae need to grow. When algae stick to those surfaces, they block out the light, requiring cleaning.
“You have to shut down and clean up the entire reactor as frequently as every two weeks,” Varanasi says. “It’s a huge operational challenge.”
The researchers realized other industries have similar problem due to many cells’ natural adhesion, or stickiness. Each industry has its own solution for cell adhesion depending on how important it is that the cells survive. Some people scrape the surfaces clean, while others use special coatings that are toxic to cells.
In the pharmaceutical and biotech industries, cell detachment is typically carried out using enzymes. However, this method poses several challenges — it can damage cell membranes, is time-consuming, and requires large amounts of consumables, resulting in millions of liters of biowaste.
To create a better solution, the researchers began by studying other efforts to clear surfaces with bubbles, which mainly involved spraying bubbles onto surfaces and had been largely ineffective.
“We realized we needed the bubbles to form on the surfaces where we don’t want these cells to stick, so when the bubbles detach it creates a local fluid flow that creates shear stress at the interface and removes the cells,” Varanasi explains.
Electric currents generate bubbles by splitting water into hydrogen and oxygen. But previous attempts at using electricity to detach cells were hampered because the cell culture mediums contain sodium chloride, which turns into bleach when combined with an electric current. The bleach damages the cells, making it impractical for many applications.
“The culprit is the anode — that’s where the sodium chloride turns to bleach,” Vandereydt explained. “We figured if we could separate that electrode from the rest of the system, we could prevent bleach from being generated.”
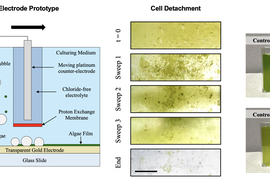
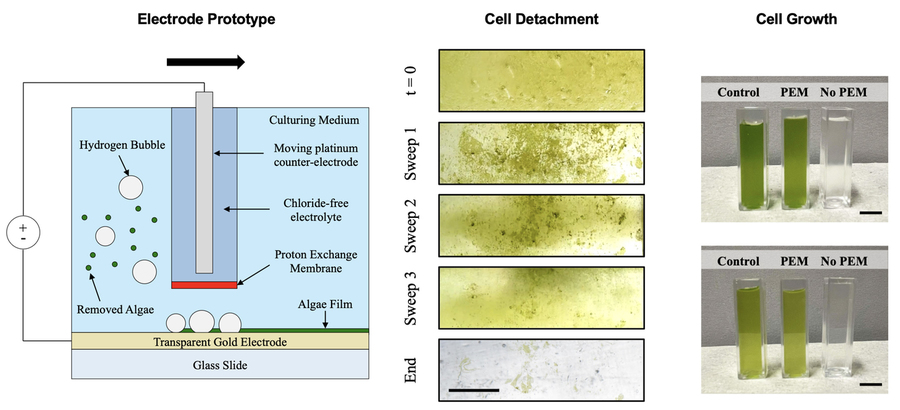
To make a better system, the researchers built a 3-square-inch glass surface and deposited a gold electrode on top of it. The layer of gold is so thin it doesn’t block out light. To keep the other electrode separate, the researchers integrated a special membrane that only allows protons to pass through. The set up allowed the researchers to send a current through without generating bleach.
To test their setup, they allowed algae cells from a concentrated solution to stick to the surfaces. When they applied a voltage, the bubbles separated the cells from the surfaces without harming them.
The researchers also studied the interaction between the bubbles and cells, finding the higher the current density, the more bubbles were created and the more algae was removed. They developed a model for understanding how much current would be needed to remove algae in different settings and matched it with results from experiments involving algae as well as cells from ovarian cancer and bones.
“Mammalian cells are orders of magnitude more sensitive than algae cells, but even with those cells, we were able to detach them with no impact to the viability of the cell,” Vandereydt says.
Getting to scale
The researchers say their system could represent a breakthrough in applications where bleach or other chemicals would harm cells. That includes pharmaceutical and food production.
“If we can keep these systems running without fouling and other problems, then we can make them much more economical,” Varanasi says.
For cell culture plates used in the pharmaceutical industry, the team envisions their system comprising an electrode that could be robotically moved from one culture plate to the next, to detach cells as they’re grown. It could also be coiled around algae harvesting systems.
“This has general applicability because it doesn’t rely on any specific biological or chemical treatments, but on a physical force that is system-agnostic,” Varanasi says. “It’s also highly scalable to a lot of different processes, including particle removal.”
Varanasi cautions there is much work to be done to scale up the system. But he hopes it can one day make algae and other cell harvesting more efficient.
“The burning problem of our time is to somehow capture CO2 in a way that’s economically feasible,” Varanasi says. “These photobioreactors could be used for that, but we have to overcome the cell adhesion problem.”
The work was supported, in part, by Eni S.p.A through the MIT Energy Initiative, the Belgian American Educational Foundation Fellowship, and the Maria Zambrano Fellowship.