Observing anything and everything within the human brain, no matter how large or small, while it is fully intact has been an out-of-reach dream of neuroscience for decades. But in a new study in Science, an MIT-based team describes a technology pipeline that enabled them to finely process, richly label, and sharply image full hemispheres of the brains of two donors — one with Alzheimer’s disease and one without — at high resolution and speed.
“We performed holistic imaging of human brain tissues at multiple resolutions, from single synapses to whole brain hemispheres, and we have made that data available,” says senior and corresponding author Kwanghun Chung, associate professor the MIT departments of Chemical Engineering and Brain and Cognitive Sciences and member of The Picower Institute for Learning and Memory and the Institute for Medical Engineering and Science. “This technology pipeline really enables us to analyze the human brain at multiple scales. Potentially this pipeline can be used for fully mapping human brains.”
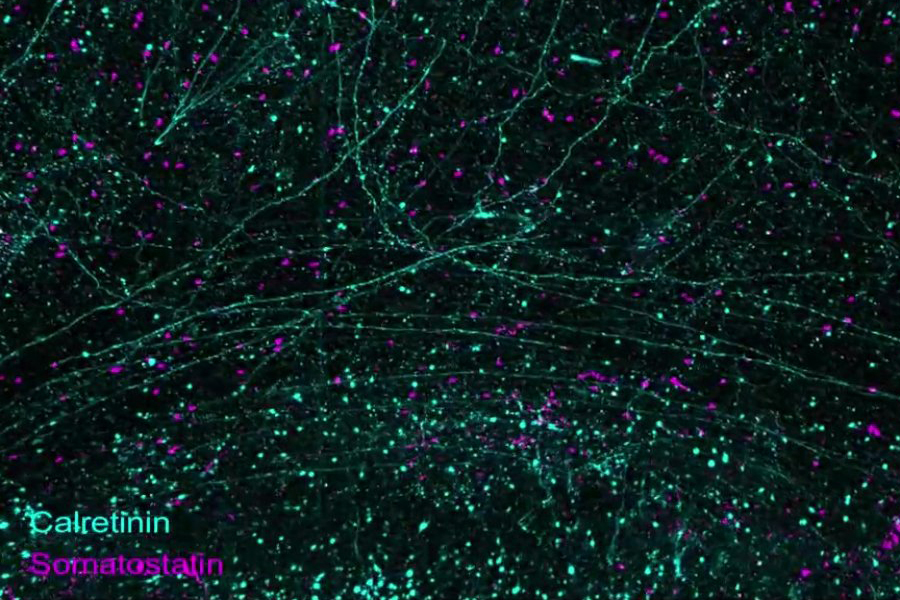
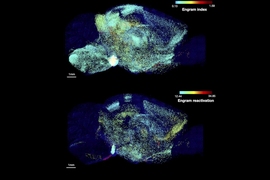
The new study does not present a comprehensive map or atlas of the entire brain, in which every cell, circuit, and protein is identified and analyzed. But with full hemispheric imaging, it demonstrates an integrated suite of three technologies to enable that and other long-sought neuroscience investigations. The research provides a “proof of concept” by showing numerous examples of what the pipeline makes possible, including sweeping landscapes of thousands of neurons within whole brain regions; diverse forests of cells, each in individual detail; and tufts of subcellular structures nestled among extracellular molecules. The researchers also present a rich variety of quantitative analytical comparisons focused on a chosen region within the Alzheimer’s and non-Alzheimer’s hemispheres.
The importance of being able to image whole hemispheres of human brains intact and down to the resolution of individual synapses (the teeny connections that neurons forge to make circuits) is two-fold for understanding the human brain in health and disease, Chung says.
Superior samples
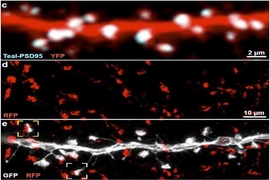
On one hand, it will enable scientists to conduct integrated explorations of questions using the same brain, rather than having to (for example) observe different phenomena in different brains, which can vary significantly, and then try to construct a composite picture of the whole system. A key feature of the new technology pipeline is that analysis doesn’t degrade the tissue. On the contrary, it makes the tissues extremely durable and repeatedly re-labelable to highlight different cells or molecules as needed for new studies for potentially years on end. In the paper, Chung’s team demonstrates using 20 different antibody labels to highlight different cells and proteins, but they are already expanding that to a hundred or more.
“We need to be able to see all these different functional components — cells, their morphology and their connectivity, subcellular architectures, and their individual synaptic connections — ideally within the same brain, considering the high individual variabilities in the human brain and considering the precious nature of human brain samples,” Chung says. “This technology pipeline really enables us to extract all these important features from the same brain in a fully integrated manner.”
On the other hand, the pipeline’s relatively high scalability and throughput (imaging a whole brain hemisphere once it is prepared takes 100 hours, rather than many months) means that it is possible to create many samples to represent different sexes, ages, disease states, and other factors that can enable robust comparisons with increased statistical power. Chung says he envisions creating a brain bank of fully imaged brains that researchers could analyze and re-label as needed for new studies to make more of the kinds of comparisons he and co-authors made with the Alzheimer’s and non-Alzheimer’s hemispheres in the new paper.
Three key innovations
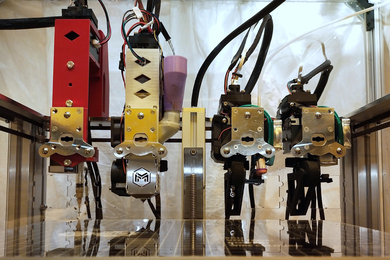
Chung says the biggest challenge he faced in achieving the advances described in the paper was building a team at MIT that included three especially talented young scientists, each a co-lead author of the paper because of their key roles in producing the three major innovations. Ji Wang, a mechanical engineer and former postdoc, developed the “Megatome,” a device for slicing intact human brain hemispheres so finely that there is no damage to them. Juhyuk Park, a materials engineer and former postdoc, developed the chemistry that makes each brain slice clear, flexible, durable, expandable, and quickly, evenly, and repeatedly labelable — a technology called “mELAST.” Webster Guan, a former MIT chemical engineering graduate student with a knack for software development, created a computational system called “UNSLICE” that can seamlessly reunify the slabs to reconstruct each hemisphere in full 3D, down to the precise alignment of individual blood vessels and neural axons (the long strands they extend to forge connections with other neurons).
No technology allows for imaging whole human brain anatomy at subcellular resolution without first slicing it, because it is very thick (it’s 3,000 times the volume of a mouse brain) and opaque. But in the Megatome, tissue remains undamaged because Wang, who is now at a company Chung founded called LifeCanvas Technologies, engineered its blade to vibrate side-to-side faster, and yet sweep wider, than previous vibratome slicers. Meanwhile she also crafted the instrument to stay perfectly within its plane, Chung says. The result are slices that don’t lose anatomical information at their separation or anywhere else. And because the vibratome cuts relatively quickly and can cut thicker (and therefore fewer) slabs of tissue, a whole hemisphere can be sliced in a day, rather than months.
A major reason why slabs in the pipeline can be thicker comes from mELAST. Park engineered the hydrogel that infuses the brain sample to make it optically clear, virtually indestructible, and compressible and expandable. Combined with other chemical engineering technologies developed in recent years in Chung’s lab, the samples can then be evenly and quickly infused with the antibody labels that highlight cells and proteins of interest. Using a light sheet microscope the lab customized, a whole hemisphere can be imaged down to individual synapses in about 100 hours, the authors report in the study. Park is now an assistant professor at Seoul National University in South Korea.
“This advanced polymeric network, which fine-tunes the physicochemical properties of tissues, enabled multiplexed multiscale imaging of the intact human brains,” Park says.
After each slab has been imaged, the task is then to restore an intact picture of the whole hemisphere computationally. Guan’s UNSLICE does this at multiple scales. For instance, at the middle, or “meso” scale, it algorithmically traces blood vessels coming into one layer from adjacent layers and matches them. But it also takes an even finer approach. To further register the slabs, the team purposely labeled neighboring neural axons in different colors (like the wires in an electrical fixture). That enabled UNSLICE to match layers up based on tracing the axons, Chung says. Guan is also now at LifeCanvas.
In the study, the researchers present a litany of examples of what the pipeline can do. The very first figure demonstrates that the imaging allows one to richly label a whole hemisphere and then zoom in from the wide scale of brainwide structures to the level of circuits, then individual cells, and then subcellular components, such as synapses. Other images and videos demonstrate how diverse the labeling can be, revealing long axonal connections and the abundance and shape of different cell types including not only neurons but also astrocytes and microglia.
Exploring Alzheimer’s
For years, Chung has collaborated with co-author Matthew Frosch, an Alzheimer’s researcher and director of the brain bank at Massachusetts General Hospital, to image and understand Alzheimer’s disease brains. With the new pipeline established they began an open-ended exploration, first noticing where within a slab of tissue they saw the greatest loss of neurons in the disease sample compared to the control. From there, they followed their curiosity — as the technology allowed them to do — ultimately producing a series of detailed investigations described in the paper.
“We didn’t lay out all these experiments in advance,” Chung says. “We just started by saying, ‘OK, let’s image this slab and see what we see.’ We identified brain regions with substantial neuronal loss so let’s see what’s happening there. ‘Let’s dive deeper.’ So we used many different markers to characterize and see the relationships between pathogenic factors and different cell types.
“This pipeline allows us to have almost unlimited access to the tissue,” Chung says. “We can always go back and look at something new.”
They focused most of their analysis in the orbitofrontal cortex within each hemisphere. One of the many observations they made was that synapse loss was concentrated in areas where there was direct overlap with amyloid plaques. Outside of areas of plaques the synapse density was as high in the brain with Alzheimer’s as in the one without the disease.
With just two samples, Chung says, the team is not offering any conclusions about the nature of Alzheimer’s disease, of course, but the point of the study is that the capability now exists to fully image and deeply analyze whole human brain hemispheres to enable exactly that kind of research.
Notably, the technology applies equally well to many other tissues in the body, not just brains.
“We envision that this scalable technology platform will advance our understanding of the human organ functions and disease mechanisms to spur development of new therapies,” the authors conclude.
In addition to Park, Wang, Guan, Chung, and Frosch, the paper’s other authors are Lars A. Gjesteby, Dylan Pollack, Lee Kamentsky, Nicholas B. Evans, Jeff Stirman, Xinyi Gu, Chuanxi Zhao, Slayton Marx, Minyoung E. Kim, Seo Woo Choi, Michael Snyder, David Chavez, Clover Su-Arcaro, Yuxuan Tian, Chang Sin Park, Qiangge Zhang, Dae Hee Yun, Mira Moukheiber, Guoping Feng, X. William Yang, C. Dirk Keene, Patrick R. Hof, Satrajit S. Ghosh, and Laura J. Brattain.
The main funding for the work came from the National Institutes of Health, The Picower Institute for Learning and Memory, The JPB Foundation, and the NCSOFT Cultural Foundation.