Researchers from the Antimicrobial Resistance (AMR) interdisciplinary research group at Singapore-MIT Alliance for Research and Technology (SMART), MIT’s research enterprise in Singapore, in collaboration with Nanyang Technological University Singapore (NTU Singapore) and National University Hospital, have discovered a novel therapy by combining two antibiotics, rifaximin and clarithromycin, to treat Mycobacterium abscessus, a non-tuberculous mycobacterium (NTM) that causes chronic lung-related infections.
Infections caused by NTM are a fast-growing health concern worldwide, particularly in the context of lung-related infections. Among NTMs, M. abscessus is one of the most prevalent, causing pulmonary infections in humans with immune deficiencies or underlying lung conditions. M. abscessus has also been linked to severe infections in various other parts of the body, including the skin, joints, soft tissues, and surgical sites. These infections are difficult to treat due to the bacterium’s extensive innate resistance to many commonly used antibiotics.
Currently, M. abscessus infections are treated by a multidrug regimen that includes clarithromycin, but some M. abscessus subspecies acquire resistance upon repeated exposure to the drug. As a result, available treatment options are limited, leading to prolonged and recurrent infections and even fatalities in some cases. With clarithromycin being the mainstay of NTM treatments and currently the only highly effective oral antibiotic for treating M. abscessus infections, there is an urgent medical need for the identification of compounds that are clarithromycin potentiators in order to effectively restore clarithromycin efficacy against M. abscessus.
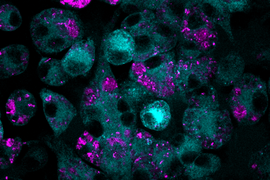
A recent open-access study by SMART researchers, “Rifaximin potentiates clarithromycin against Mycobacterium abscessus in vitro and in zebrafish,” published in the scientific journal JAC-Antimicrobial Resistance, revealed promising findings on the use of rifaximin, an antibiotic commonly used to treat gastrointestinal bacterial infections, as a clarithromycin potentiator with the ability to increase clarithromycin sensitivity and improve its ability to kill M. abscessus. During the discovery stage of the study, the researchers conducted drug screening campaigns and successfully identified several drug candidates as clarithromycin potentiators. Further preclinical testing of these drug candidates confirmed rifaximin as the most effective clarithromycin potentiator, with the combination of rifaximin and clarithromycin showing efficacy both in vitro and in a zebrafish embryo infection model.
“With limited treatments available due to M. abscessus's innate resistance to most antibiotics, including clarithromycin, the novel discovery of the strong combination between rifaximin and clarithromycin is a significant step towards addressing the challenge of treating NTM infections. As FDA-approved drugs, we will be able to quicken the process and translate the findings into improved treatment outcomes for patients suffering from M. abscessus infections,” says MIT professor of biological engineering Peter C. Dedon, senior author of the paper and co-lead principal investigator at SMART AMR.
“We recognize the urgent need to address the growing problem of clarithromycin resistance in M. abscessus and are pleased to have discovered rifaximin as a potent clarithromycin potentiator. Despite being primarily used for gastrointestinal infections and having limited activity against drug-resistant M. abscessus, our study demonstrated the synergistic effect of rifaximin with clarithromycin in effectively eliminating the bacteria,” adds Boon Chong Goh, first author of the paper and principal research scientist at SMART AMR.
The researchers are furthering their research with animal preclinical studies to prepare for human clinical trials. As both rifaximin and clarithromycin are approved by the U.S. Food and Drug Administration, the preclinical studies evaluating their combination against M. abscessus can be accelerated. The team is also collaborating with a commercial manufacturing partner to create inhalation formulations suitable for delivering the drug combination directly to the lungs for use in human clinical trials.
The research was carried out by SMART and supported by the A*STAR Singapore Therapeutics Development Review, the SMART Innovation Centre, and the National Research Foundation (NRF) Singapore under its Campus for Research Excellence And Technological Enterprise (CREATE) program. The NTU Singapore researchers played an important role in performing the zebrafish embryo infection model, and NUH provided the clinical isolates of M. abscessus.
The AMR IRG is a translational research and entrepreneurship program that tackles the growing threat of antimicrobial resistance. By leveraging talent and convergent technologies across Singapore and MIT, they tackle AMR head-on by developing multiple innovative and disruptive approaches to identify, respond to, and treat drug-resistant microbial infections. Through strong scientific and clinical collaborations, they provide transformative, holistic solutions for Singapore and the world. SMART was established by MIT and the NRF is 2007. SMART is the first entity in CREATE, undertaking cutting-edge research in areas of interest to both Singapore and MIT.