From steering a car to swinging a tennis racket, we learn to execute all kinds of skilled movements during our lives. You might think this learning is only implemented by neurons, but a new study by researchers at The Picower Institute for Learning and Memory at MIT shows the essential role of another brain cell type: astrocytes.
Just as teams of elite athletes train alongside staffs of coaches, ensembles of neurons in the brain’s motor cortex depend on nearby astrocytes to help them learn to encode when and how to move, and the optimal timing and trajectory of a motion, the study shows. Describing a series of experiments in mice, the new paper in the Journal of Neuroscience reveals two specific ways that astrocytes directly impact motor learning, maintaining an optimal molecular balance in which the neuronal ensembles can properly refine movement performance.
“This finding is part of a body of work from our lab and other labs that elevate the importance of astrocytes to neuronal encoding and hence to behavior,” says senior author Mriganka Sur, the Newton Professor of Neuroscience in The Picower Institute and MIT’s Department of Brain and Cognitive Sciences. “This shows that while the population coding of behaviors is a neuronal function, we need to include astrocytes as partners with them.”
Picower Institute postdoc Jennifer Shih and former Sur Lab postdocs Chloe Delepine and Keji Li are the paper’s co-lead authors.
“This research highlights the complexity of astrocytes and the importance of astrocyte-neuron interactions in fine-tuning brain function by providing concrete evidence of these mechanisms in the motor cortex,” Delepine says.
The team gave their mice a simple motor task to master. When cued with a tone, the mice had to reach for and push down a lever within five seconds. The rodents showed they could learn the task over a few days and master it within a couple of weeks. They not only performed the task more accurately, but also their reactions quickened and the trajectory of their reaching and pushing became smoother and more uniform.
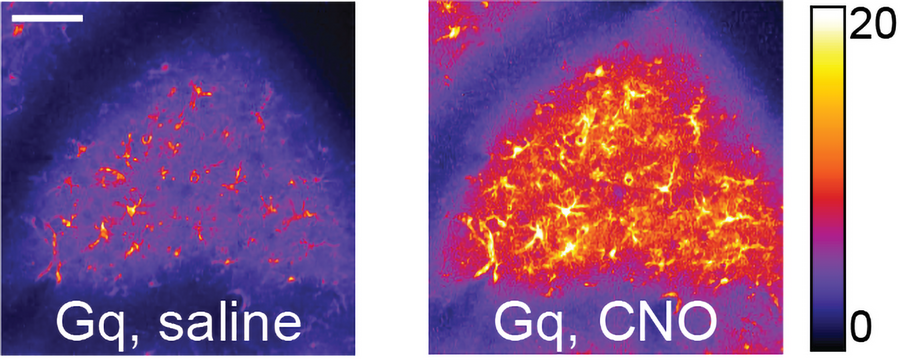
In some of the mice, however, the team employed precision molecular interventions to disrupt two specific functions of astrocytes in the motor cortex. In some mice, they disrupted the astrocytes’ ability to soak up the neurotransmitter glutamate, a chemical that excites neural activity when it is received at connections called synapses. In other mice they hyperactivated the astrocytes’ calcium signals, which affected how they function. In both ways, the interventions disrupted the normal process by which neurons would form or change their connections with each other, a process called “plasticity” that enables learning.
The interventions each affected the performance of the mice. The first one (a knockdown of the glutamate transporter GLT1) didn’t affect whether the mice pushed the lever or how quickly they did so. Instead it disrupted the smoothness of the motion. Mice with GLT1 disrupted remained erratic and shaky, as if unable to refine their technique. Mice subjected to the second intervention (activation of Gq signaling) showed deficits not only in the smoothness of their motion trajectory but also in their understanding of when to push the lever and their quickness in doing so.
The team dug deeper into how these deficits emerged. Using a two-photon microscope, they tracked neural activity in the motor cortex in unaltered mice and mice treated with each intervention. Compared to what they saw in normal mice, the mice with GLT1 disrupted showed less correlated activity among neurons. Mice with Gq activation showed excessive correlated activity compared to the normal mice.
“The data suggest that an optimal level of neuronal correlation is required for the emergence of functional neuronal ensembles that drive task performance,” the authors wrote. “Meaningful correlations that carry information are what drive motor learning, rather than the absolute magnitude of potentially nonspecific correlations.”
The team dug even deeper still. They carefully isolated astrocytes from the motor cortex of mice, including some who were untrained in the motor task as well as ones who were trained, including mice who were unaltered and mice who underwent each intervention. In all these samples of purified astrocytes, they then sequenced RNA to assess how they differed in their expression of genes. They found that in trained versus untrained mice, astrocytes exhibited greater expression of genes related to GLT1. In mice where they intervened they saw lowered expression. That evidence further suggested that the glutamate transporter process is indeed fundamental to training in motor tasks.
“Here we show that astrocytes have an important role in enabling neurons to encode information properly, both the learning and the execution of a movement, for example,” Sur says.
Pierre Gaudeaux is a co-author of the paper. The research was funded by The National Institutes of Health, the Simons Foundation, and The JPB Foundation.