Researchers from the Antimicrobial Resistance (AMR) Interdisciplinary Research Group at Singapore-MIT Alliance for Research and Technology (SMART), MIT’s research enterprise in Singapore, alongside collaborators at Nanyang Technological University, have identified a novel phage lysin — Abp013 — that could be used as an alternative antimicrobial agent against two of the most deadly bacteria: Acinetobacter baumannii and Klebsiella pneumoniae.
Lysins — enzymes produced by bacteriophages — have displayed great potential as a novel class of antimicrobials as their properties allow them to quickly and directly target key structural components of a bacteria’s cell walls, and in doing so, reduce the bacteria’s ability to develop resistance.
The inappropriate and extensive use of antibiotics over the past few decades has led to the emergence of antimicrobial resistance — a phenomenon in which bacterial strains develop mechanisms to resist medicines designed to kill them. In 2019 alone, it is estimated that 4.95 million people died from infections either associated with or attributable to antimicrobial resistance. This already pressing issue, compounded further by the widespread usage of antibiotics during the Covid-19 pandemic, highlights the urgent need for new therapeutic agents that are difficult for bacteria to develop resistance against.
“Antimicrobial resistance remains an ever-growing threat to humankind, and an increasing number of people die each year from superbug infections. The development of new bacteria-killing agents is crucial, and lysins have shown great promise in treating deadly chronic wound and lung infections against which no antibiotics are effective and limited treatment options are available,” says Joash Chu, first author of the paper that documented the discovery, and a researcher at SMART at the time of the study.
Lysins have been highly effective in fighting Gram-positive bacteria — which do not have an outer lipid membrane and are thus easily killed by lysins. Conversely, in Gram-negative bacteria, the presence of an outer membrane impedes many lysins from killing the bacteria efficiently. Hence, the discovery of novel lysin Abp013 is crucial in advancing treatment methods against multidrug-resistant Gram-negative pathogens.
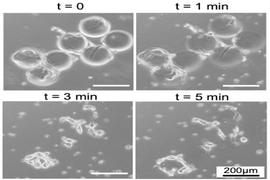
In a paper titled “Novel Phage Lysin Abp013 against Acinetobacter baumannii” published in medical journal Antibiotics, the SMART AMR team reveals their findings on Abp013’s ability to effectively access and kill various bacterial strains. The study showed that Abp013 displayed good permeability and killing activity against multiple Acinetobacter baumannii and Klebsiella pneumoniae strains, even when they are in a more complex environment in which typical lysins are ineffective.
Acinetobacter baumannii and Klebsiella pneumoniae are superbugs responsible for a multitude of potentially life-threatening infections, such as pneumonia and meningitis, especially among the ill and immunocompromised. Unfortunately, many strains of these bacteria are difficult to treat as they grow increasingly resistant to antibiotics. Typically, to treat Acinetobacter infections, health-care providers have to send a specimen for laboratory testing to determine which antibiotics are effective in fighting the bacteria. Thus, the important discovery of Abp013 and its unique bacteria-targeting properties could advance the development of remedies for quicker and more effective targeting of these bacteria.
“Abp013 is the first Gram-negative lysin found to display host selectivity. Prior to the discovery of Abp013, no other lysins are capable of targeting Acinetobacter baumannii and Klebsiella pneumoniae, but not Pseudomonas aeruginosa. Understanding the mechanism behind such selectivity will help guide the development of lysin variants customized to only target pathogenic bacteria, for more precise treatment of bacterial infections,” says Goh Boon Chong, principal research scientist at SMART AMR, and a co-corresponding author of the paper.
Moving forward, the researchers will further investigate the crystal structure of this novel lysin and understand its unique underlying mechanisms. These will open the possibility of swapping or merging the lysin’s components with other lysins or antimicrobial components to spur the engineering of Gram-negative lysins with superior potency, and lead to the development of alternative therapeutic agents that can resist the resistance.
SMART AMR has been developing methods for the customized targeting of bacteria using lysins, and these endeavors were nurtured by the early support of the SMART Innovation Centre. National Research Foundation (NRF) Singapore’s Intra-CREATE Collaborative Seed Grant encourages and facilitates collaboration between CREATE’s partner institutions co-located in Singapore, to achieve greater impact from collaborative research efforts.
SMART was established by MIT and the NRF in 2007. SMART is the first entity in the Campus for Research Excellence and Technological Enterprise (CREATE) and serves as an intellectual and innovation hub for cutting-edge research interactions between MIT and Singapore. SMART currently comprises an Innovation Centre and five Interdisciplinary Research Groups (IRGs): AMR, Critical Analytics for Manufacturing Personalized-Medicine, Disruptive & Sustainable Technologies for Agricultural Precision, Future Urban Mobility, and Low Energy Electronic Systems.
SMART research is funded by the NRF under the CREATE program. This study is supported by the NRF, under its Intra-CREATE Collaborative Seed Grant.
The AMR IRG is a translational research and entrepreneurship program that tackles the growing threat of antimicrobial resistance. By leveraging talent and convergent technologies across Singapore and MIT, AMR develops multiple innovative and disruptive approaches to identify, respond to, and treat drug-resistant microbial infections. Through strong scientific and clinical collaborations, their goal is to provide transformative, holistic solutions for Singapore and the world.