Even in Earth’s most inhospitable environments, life has taken hold.
Extremophiles are the organisms most well-known for withstanding extreme temperatures, pHs, salinity, and even nutrient-starvation. They have evolved special mechanisms that enable them to survive in their environments, but getting to the bottom of that resilience requires targeted and methodical interrogation.
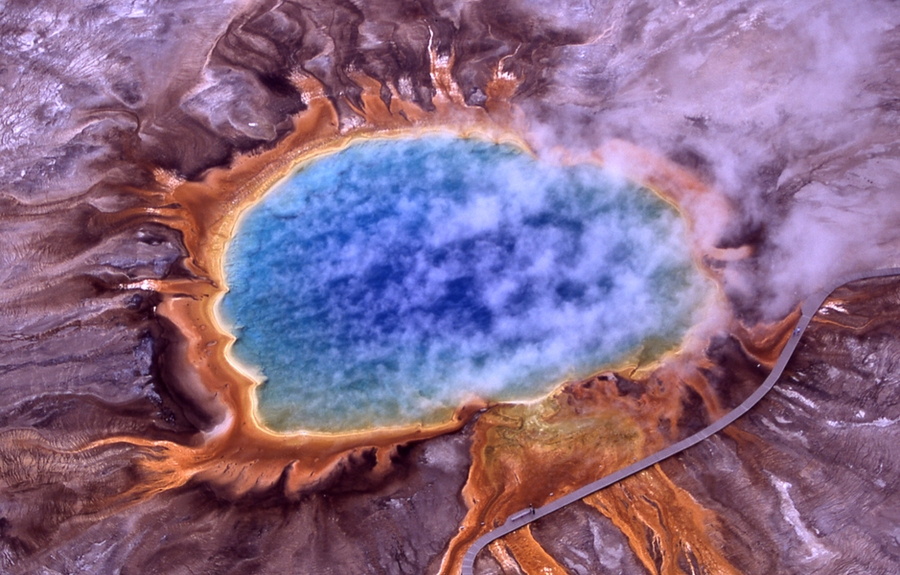
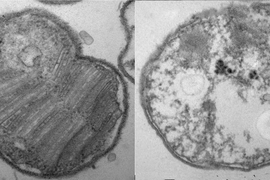
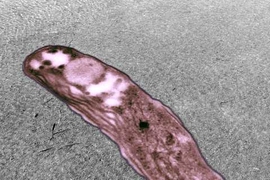
At Yellowstone National Park and similar sites, extremophiles reside in environments such as acid hot springs or thermal acid soils. Here they are exposed, often intermittently, to some of the lowest naturally-occurring pHs on Earth, and temperatures nearing the boiling point of water. To survive in these rapidly fluctuating conditions, organisms protect themselves with complex membranes, composed of interlocked lipids linked to their backbones with strong ether bonds, rather than the ester bonds most commonly found in eukaryotes and bacteria.
In Sulfolobus acidocaldarius, an archaeon that lives in high-acid, high-temperature environments that are common in Yellowstone, cellular membrane lipids called glycerol dialkyl glycerol tetraether (GDGTs) are linked to an uncommon sugar-like molecule called calditol. A group of scientists recently published findings in the Proceedings of the National Academy of Sciences (PNAS), identifying how calditol is made in the cell and how, specifically, it is responsible for acid-tolerance in these organisms. The work is helping scientists get closer to understanding how life evolved to survive in extreme environments.
Roger Summons, the Schlumberger Professor of Geobiology in MIT’s Department of Earth, Atmospheric and Planetary Sciences (EAPS) and one of the authors of the study, credits advances in molecular biology, bioinformatics, and targeted gene deletion strategies for enabling this discovery.
“The era of genomics has brought a range of new tools to advance lipid biomarker research,” Summons says. Paula Welander, a former EAPS postdoc in the Summons Lab and now assistant professor in the Department of Earth System Science at Stanford University, directed the study that was also conducted by Zhirui Zeng and Jeremy H. Wei at Stanford, and Xiao-lei Liu, an assistant professor of organic geochemistry at the University of Oklahoma.
“This study is an excellent example of how an interdisciplinary approach, including microbial physiologists and organic geochemists, can address outstanding questions regarding lipid biomarkers,” Welander says.
To identify calditol’s role in the Sulfolobus acidocaldarius membranes, the researchers used tools in comparative genomics, gene deletion, and lipid analysis to zero in on a particular protein within the class of radical S-andenosylmethionine (SAM) enzymes that is required to synthesize calditol. When they searched for what coded that protein in calditol-producing archaeal genomes, they found just a few candidate genes.
To test the protein’s importance for acid tolerance, the researchers created mutants — with the membrane-related genes deleted — and analyzed their lipids. By subjecting the calditol-free mutant to highly acidic conditions, the researchers were able to confirm the true function of the calditol component of the membrane. Only the naturally-occurring, calditol-producing Sulfolobus and the mutant strain with the radical-SAM gene restored, were able to grow after a significant drop in pH.
“While Welander and colleagues have demonstrated the presence of radical-SAM lipid biosynthesis genes in bacteria, this is the first time one has been unambiguously identified in archaea,” Summons says. “Calditol-linked to membrane lipids in these organisms confer significant protective effects.”
Adds Welander: “Researchers have hypothesized for many years that producing calditol would provide this type of protective effect, but this has not been demonstrated directly. Here we finally show this link directly.”
Even further, the fact that a radical SAM protein is involved in linking calditol to the membranes might help scientists better understand the chemistry and evolution of membrane lipids from a wide variety of environments across the planet.
Summons says the result speaks to “the possible presence of a variety of other radical chemistries to modify membrane lipids once they’ve been synthesized.”
“In turn, this could help us better understand the biosynthesis of other archaea-specific lipids and help us write the evolutionary history of these strikingly distinctive membranes,” he says.
The study was supported by the Simons Foundation Collaboration on the Origins of Life.