The reluctance of oil and water to mix together and stay that way is so well-known that it has become a cliché for describing any two things that do not go together well. Now, a new finding from researchers at MIT might turn that expression on its head, providing a way to get the two substances to mix and remain stable for long periods — no shaking required. The process may find applications in pharmaceuticals, cosmetics, and processed foods, among other areas.
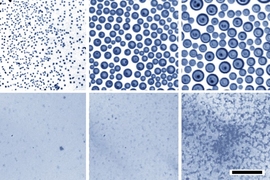
The new process involves cooling a bath of oil containing a small amount of a surfactant (a soap-like substance), and then letting water vapor from the surrounding air condense onto the oil surface. Experiments have shown that this can produce tiny, uniform water droplets on the surface that then sink into the oil, and their size can be controlled by adjusting the proportion of surfactant. The findings, by MIT graduate student Ingrid Guha, former postdoc Sushant Anand, and associate professor Kripa Varanasi, are reported in the journal Nature Communications.
As anyone who has ever used salad dressing knows, no matter how vigorously the mixture gets shaken, the oil and the vinegar (a water-based solution) will separate within minutes. But for many uses, including new drug-delivery systems and food-processing methods, it’s important to be able to get oil in water (or water in oil) to form tiny droplets — only a few hundred nanometers across, too small to see with the naked eye — and to have them stay tiny rather than coalescing into larger droplets and eventually separating from the other liquid.
Typically, in industrial processes these emulsions are made by either mechanically shaking the mix or using sound waves to set up intense vibrations within the liquid, a process called sonicating. But both of these processes “require a lot of energy,” Varanasi says, “and the finer the drops, the more energy it takes.” By contrast, “our approach is very energy inexpensive,” he adds.
“The key to overcoming that separation is to have really small, nanoscale droplets,” Guha explains. “When the drops are small, gravity can’t overcome them,” and they can remain suspended indefinitely.
For the new process, the team set up a reservoir of oil with an added surfactant that can bind to both oil and water molecules. They placed this inside a chamber with very humid air and then cooled the oil. Like a glass of cold water on a hot summer day, the colder surface causes the water vapor to precipitate. The condensing water then forms droplets at the surface that spread through the oil-surfactant mixture, and the sizes of these droplets are quite uniform, the team found. “If you get the chemistry just right, you can get just the right dispersion,” Guha says. By adjusting the proportion of surfactant in the oil, the droplet sizes can be well-controlled.
In the experiments, the team produced nanoscale emulsions that remained stable over periods of several months, compared to the few minutes that it takes for the same mixture of oil and water to separate without the added surfactant. “The droplets stay so small that they’re hard to see even under a microscope,” Guha says.
Unlike the shaking or sonicating methods, which take the large, separate masses of oil and water and gradually get them to break down into smaller drops — a “top down” approach — the condensation method starts off right away with the tiny droplets condensing out from the vapor, which the researchers call a bottom-up approach. “By cloaking the freshly condensed nanoscale water droplets with oil, we are taking advantage of the inherent nature of phase-change and spreading phenomena,” Varanasi says.
“Our bottom-up approach of creating nanoscale emulsions is highly scalable owing to the simplicity of the process,” Anand says. “We have uncovered many new phenomena during this work. We have found how the presence of surfactant can change the oil and water interactions under such conditions, promoting oil spreading on water droplets and stabilizing them at the nanoscale.”
The team says that the approach should work with a variety of oils and surfactants, and now that the process has been identified, their findings “provide a kind of design guideline for someone to use” for a particular kind of application, Varanasi says.
“It’s such an important thing,” he says, because “foods and pharmaceuticals always have an expiration date,” and often that has to do with the instability of the emulsions in them. The experiments used a particular surfactant that is widely used, but many other varieties are available, including some that are approved for food-grade products.
In addition, Guha says, “we envision that you could use multiple liquids and make much more complex emulsions.” And besides being used in food, cosmetics, and drugs, the method could have other applications, such as in the oil and gas industry, where fluids such as the drilling “muds” sent down wells are also emulsions, Varanasi says.
The work was supported by the MIT Energy Initiative, the National Science Foundation, and a Society in Science fellowship. Anand, the co-author who was a postdoc at MIT, is now an assistant professor at the University of Illinois.