New treatments based on biological molecules like RNA give scientists unprecedented control over how cells function. But delivering those drugs to the right tissues remains one of the biggest obstacles to turning these promising yet fragile molecules into powerful new treatments.
Now Gensaic, founded by Lavi Erisson MBA ’19; Uyanga Tsedev SM ’15, PhD ’21; and Jonathan Hsu PhD ’22, is building an artificial intelligence-powered discovery engine to develop protein shuttles that can deliver therapeutic molecules like RNA to specific tissues and cells in the body. The company is using its platform to create advanced treatments for metabolic diseases and other conditions. It is also developing treatments in partnership with Novo Nordisk and exploring additional collaborations to amplify the speed and scale of its impact.
The founders believe their delivery technology — combined with advanced therapies that precisely control gene expression, like RNA interference (RNAi) and small activating RNA (saRNA) — will enable new ways of improving health and treating disease.
“I think the therapeutic space in general is going to explode with the possibilities our approach unlocks,” Erisson says. “RNA has become a clinical-grade commodity that we know is safe. It is easy to synthesize, and it has unparalleled specificity and reversibility. By taking that and combining it with our targeting and delivery, we can change the therapeutic landscape.”
Drinking from the firehose
Erisson worked on drug development at the large pharmaceutical company Teva Pharmaceuticals before coming to MIT for his Sloan Fellows MBA in 2018.
“I came to MIT in large part because I was looking to stretch the boundaries of how I apply critical thinking,” Erisson says. “At that point in my career, I had taken about 10 drug programs into clinical development, with products on the market now. But what I didn’t have were the intellectual and quantitative tools for interrogating finance strategy and other disciplines that aren’t purely scientific. I knew I’d be drinking from the firehose coming to MIT.”
Erisson met Hsu and Tsedev, then PhD students at MIT, in a class taught by professors Harvey Lodish and Andrew Lo. The group started holding weekly meetings to discuss their research and the prospect of starting a business.
After Erisson completed his MBA program in 2019, he became chief medical and business officer at the MIT spinout Iterative Health, a company using AI to improve screening for colorectal cancer and inflammatory bowel disease that has raised over $200 million to date. There, Erisson ran a 1,400-patient study and led the development and clearance of the company’s software product.
During that time, the eventual founders continued to meet at Erisson’s house to discuss promising research avenues, including Tsedev’s work in the lab of Angela Belcher, MIT’s James Mason Crafts Professor of Biological Engineering. Tsedev’s research involved using bacteriophages, which are fast-replicating protein particles, to deliver treatments into hard-to-drug places like the brain.
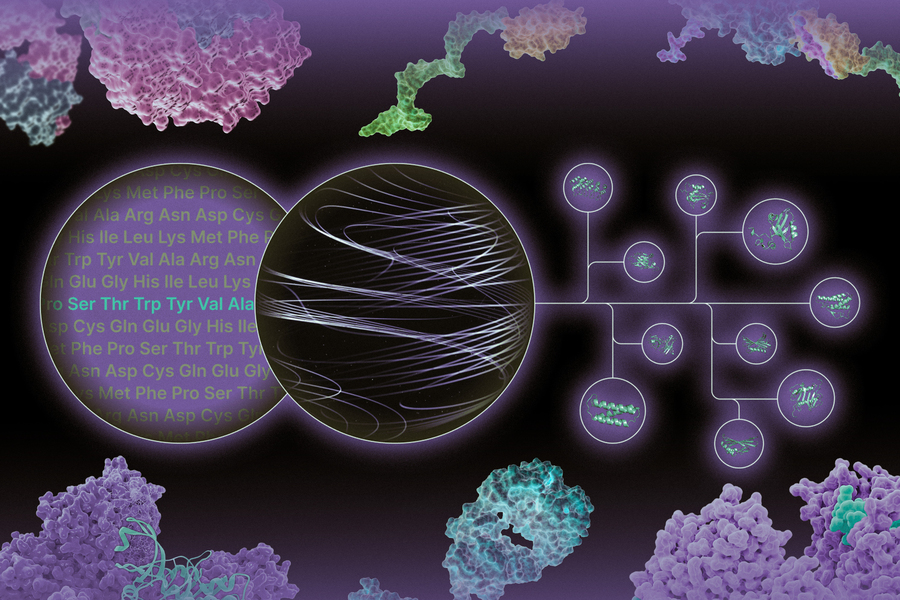
As Hsu and Tsedev neared completion of their PhDs, the team decided to commercialize the technology, founding Gensaic at the end of 2021. Gensaic’s approach uses a method called unbiased directed evolution to find the best protein scaffolding to reach target tissues in the body.
“Directed evolution means having a lot of different species of proteins competing together for a certain function,” Erisson says. “The proteins are competing for the ability to reach the right cell, and we are then able to look at the genetic code of the protein that has ‘won’ that competition. When we do that process repeatedly, we find extremely adaptable proteins that can achieve the function we’re looking for.”
Initially, the founders focused on developing protein scaffolds to deliver gene therapies. Gensaic has since pivoted to focus on delivering molecules like siRNA and RNAi, which have been hard to deliver outside of the liver.
Today Gensaic has screened more than 500 billion different proteins using a process called phage display and directed evolution. It calls its platform FORGE, for Functional Optimization by Recursive Genetic Evolution.
Erisson says Gensaic’s delivery vehicles can also carry multiple RNA molecules into cells at the same time, giving doctors a novel and powerful set of tools to treat and prevent diseases.
“Today FORGE is built into the idea of multifunctional medicines,” Erisson says. “We are moving into a future where we can extract multiple therapeutic mechanisms from a single molecule. We can combine proteins with multiple tissue selectivity and multiple molecules of siRNA or other therapeutic modalities, and affect complex disease system biology with a single molecule.”
A “universe of opportunity”
The founders believe their approach will enable new ways of improving health by delivering advanced therapies directly to new places in the body. Precise delivery of drugs to anywhere in the body could not only unlock new therapeutic targets but also boost the effectiveness of existing treatments and reduce side effects.
“We’ve found we can get to the brain, and we can get to specific tissues like skeletal and adipose tissue,” Erisson says. “We’re the only company, to my knowledge, that has a protein-based delivery mechanism to get to adipose tissue.”
Delivering drugs into fat and muscle cells could be used to help people lose weight, retain muscle, and prevent conditions like fatty liver disease or osteoporosis.
Erisson says combining RNA therapeutics is another differentiator for Gensaic.
“The idea of multiplexed medicines is just emerging,” Erisson says. “There are no clinically approved drugs using dual-targeted siRNAs, especially ones that have multi-tissue targeting. We are focused on metabolic indications that have two targets at the same time and can take on unique tissues or combinations of tissues.”
Gensaic’s collaboration with Novo Nordisk, announced last year, targets cardiometabolic diseases and includes up to $354 million in upfront and milestone payments per disease target.
“We already know we can deliver multiple types of payloads, and Novo Nordisk is not limited to siRNA, so we can go after diseases in ways that aren’t available to other companies,” Erisson says. “We are too small to try to swallow this universe of opportunity on our own, but the potential of this platform is incredibly large. Patients deserve safer medicines and better outcomes than what are available now.”