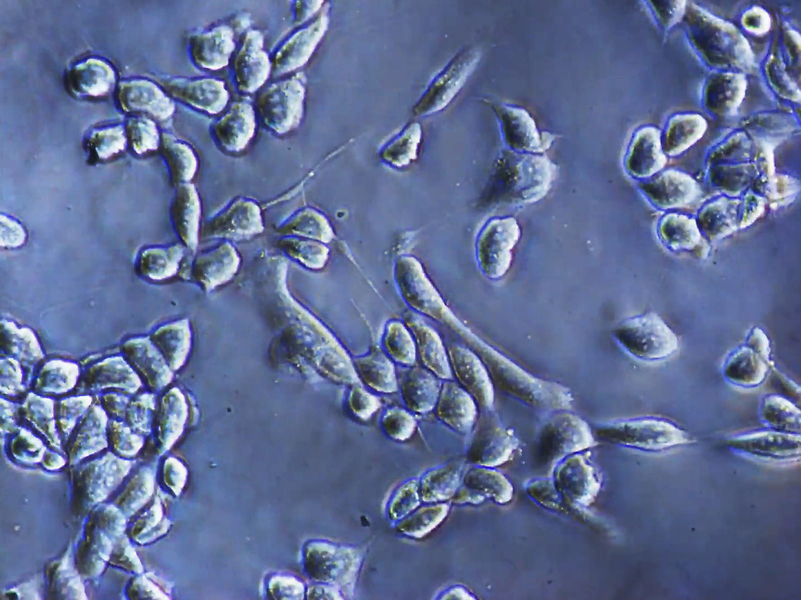
Anchorage-dependent cells are cells that require physical attachment to a solid surface, such as a culture dish, to survive, grow, and reproduce. In the biomedical industry, and others, having the ability to culture these cells is crucial, but current techniques used to separate cells from surfaces can induce stresses and reduce cell viability.
“In the pharmaceutical and biotechnology industries, cells are typically detached from culture surfaces using enzymes — a process fraught with challenges,” says Kripa Varanasi, MIT professor of mechanical engineering. “Enzymatic treatments can damage delicate cell membranes and surface proteins, particularly in primary cells, and often require multiple steps that make the workflow slow and labor-intensive.”
Existing approaches also rely on large volumes of consumables, generating an estimated 300 million liters of cell culture waste each year. Moreover, because these enzymes are often animal-derived, they can introduce compatibility concerns for cells intended for human therapies, limiting scalability and high-throughput applications in modern biomanufacturing.
Varanasi is corresponding author on a new paper in the journal ACS Nano, in which researchers from the MIT Department of Mechanical Engineering and the Cancer Program at the Broad Institute of Harvard and MIT present a novel enzyme-free strategy for detaching cells from culture surfaces. The method works by harnessing alternating electrochemical current on a conductive biocompatible polymer nanocomposite surface.
“By applying low-frequency alternating voltage, our platform disrupts adhesion within minutes while maintaining over 90 percent cell viability — overcoming the limitations of enzymatic and mechanical methods that can damage cells or generate excess waste,” says Varanasi.
Beyond simplifying routine cell culture, the approach could transform large-scale biomanufacturing by enabling automated and contamination-conscious workflows for cell therapies, tissue engineering, and regenerative medicine. The platform also provides a pathway for safely expanding and harvesting sensitive immune cells for applications such as CAR-T therapies.
“Because our electrically tunable interface can dynamically shape the ionic microenvironment around cells, it also offers powerful opportunities to control ion channels, study signaling pathways, and integrate with bioelectronic systems for high-throughput drug screening, regenerative medicine, and personalized therapies,” Varanasi explains.
“Our work shows how electrochemistry can be harnessed not just for scientific discovery, but also for scalable, real-world applications,” says Wang Hee (Wren) Lee, MIT postdoc and co-first author. “By translating electrochemical control into biomanufacturing, we’re laying the foundation for technologies that can accelerate automation, reduce waste, and ultimately enable new industries built on sustainable and precise processing.”
Bert Vandereydt, co-first author and mechanical engineering researcher at MIT, emphasizes the potential for industrial scalability. “Because this method can be applied uniformly across large areas, it’s ideal for high-throughput and large-scale applications like cell therapy manufacturing. We envision it enabling fully automated, closed-loop cell culture systems in the near future.”
Yuen-Yi (Moony) Tseng, principal investigator at the Broad Institute and collaborator on the project, underscores the biomedical significance. “This platform opens new doors for culturing and harvesting delicate primary or cancer cells. It could streamline workflows across research and clinical biomanufacturing, reducing variability and preserving cell functionality for therapeutic use.”
Industrial applications of adherent cells include uses in the biomedical, pharmaceutical, and cosmetic sectors. For this study, the team tested their new method using human cancer cells, including osteosarcoma and ovarian cancer cells. After identifying an optimal frequency, the detachment efficiency for both types of cells increased from 1 percent to 95 percent, with cell viability exceeding 90 percent.
The paper, “Alternating Electrochemical Redox-Cycling on Nanocomposite Biointerface for High-Efficiency Enzyme-Free Cell Detachment,” is available from the American Chemical Society journal ACS Nano.