On a summer evening in 1973, Richard Schrock came home from his job at DuPont’s Central Research Department and told his wife, Nancy, “I think I’ve done something important.”
As part of his research exploring synthesis of new polymers, Schrock had created a novel type of molecule with a double bond between a metal and a carbon atom. This type of compound, known as an alkylidene, had never been seen before.
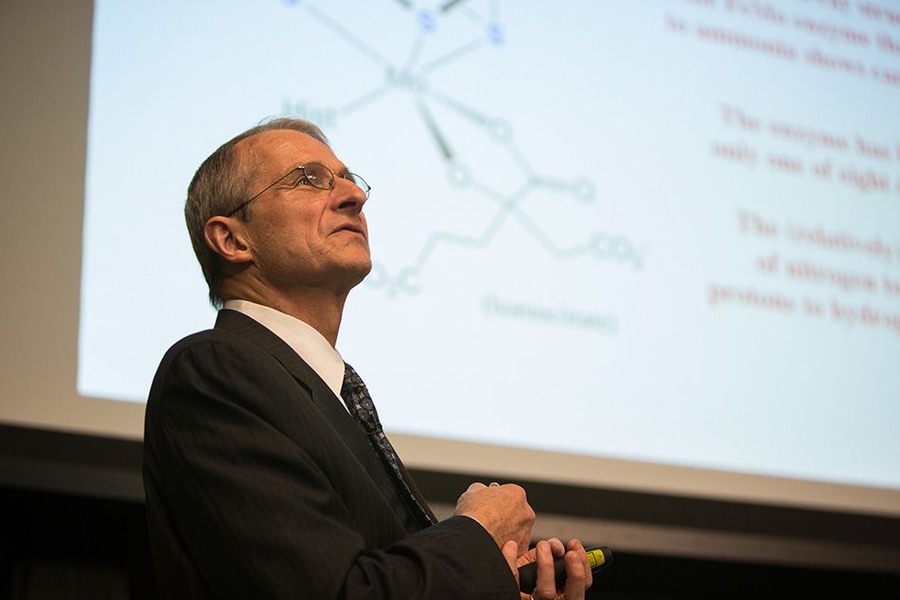
Schrock, this year’s recipient of the James R. Killian Jr. Faculty Achievement Award, described at yesterday’s Killian Lecture how that discovery set him on a path that would ultimately lead to the development of catalysts that can control the formation of many kinds of organic compounds. This work, which has been applied in the chemical industry to efficiently produce pharmaceuticals, plastics, fuels, and other substances, also earned Schrock the Nobel Prize in Chemistry in 2005.
Established in 1971 to honor MIT’s 10th president, the Killian Award recognizes extraordinary professional achievements by an MIT faculty member. The award citation noted that Schrock, the Frederick G. Keyes Professor of Chemistry at MIT, has also made valuable contributions to MIT’s educational mission: He has mentored more than 185 graduate students and postdocs, and continues to serve as a lecturer in MIT’s freshman chemistry course.
“He began this challenging teaching assignment in the early 1990s and returned to it after winning the Nobel Prize, inspiring undergraduates to unravel the beauty of molecular structures and chemical reactions,” said Susan Silbey, chair of the MIT faculty, in presenting the award before yesterday’s lecture.
“Magical little machines”
Schrock’s interest in chemistry was kindled at age 8, when his older brother, Ted, gave him a chemistry set. After earning his bachelor’s degree in chemistry from the University of California at Riverside, he did his graduate studies at Harvard University, where he worked in the lab of inorganic chemist John Osborn.
“John got me interested in catalysis,” Schrock recalled. “Catalysts are these magical little machines that can make something over and over again.”
After earning his PhD, Schrock spent a year at Cambridge University, where he met a scientist on sabbatical from DuPont, and that connection eventually led to a job offer for Schrock. When he got there, “They told me, why don’t you go make a new polymer?” he said. “That means you’ve got to make a new catalyst.”
DuPont had done some previous work using the metals chromium, vanadium, and titanium as catalysts, but Schrock turned his attention to tantalum. That summer in 1973, he realized that he had created a compound containing a double bond between tantalum and carbon.
Schrock joined the MIT faculty in 1975, and through further research there he uncovered the role that these metal-carbon bonds play in a type of reaction known as olefin metathesis. This reaction had been first seen in the 1950s but was not well understood.
Yves Chauvin, who shared the 2005 Nobel Prize with Schrock, first proposed the mechanism for this kind of reaction in 1971. Olefin metathesis involves breaking and making double bonds between carbon atoms, with help from a catalyst that contains a metal-carbon double bond, which forms a ring that contains the metal and three carbon atoms.
In 1986, Schrock developed the first catalyst that could perform this type of reaction: an atom of the metal molybdenum attached to organic structures known as ligands. He won the Nobel Prize for this work in 2005 but said yesterday, “At the time of the Nobel Prize, the most important problems in this area weren’t solved.”
One major issue to be resolved was how to control the configuration of the olefin products, which can occur in one of two configurations. Since 2005, Schrock has developed new catalysts that can control these configurations, making it easier for chemists to design possible new drugs and other useful compounds.
There is always room for improvement in designing new catalysts and reactions, noted Schrock, who described himself as “a molecular engineer.”
“Once you have a reaction that you know works, you want to build on it, tinker with it, and make it go in the direction you want it to go,” he said.
Beyond Haber-Bosch
Schrock also described his other major research focus, which centers on nitrogen. Nitrogen, found in proteins, DNA, and RNA, is essential for all life on Earth, but for most organisms to use it, it has to be converted to ammonia. Many microbes can perform this conversion, and in the early 1900s, scientists began seeking their own methods.
Two German scientists, Fritz Haber and Carl Bosch, developed a chemical process that is now used to produce 300 million tons of ammonia every year, for use in fertilizer and other compounds. It is estimated that 1.4 percent of all energy used by humans goes into producing ammonia through this process, in part because the reaction must be performed at very high temperatures and pressures.
The process also releases carbon dioxide as a byproduct, so Schrock and others have been seeking alternative ways to convert nitrogen to ammonia.
In a landmark 2003 Science paper, Schrock reported that using a molybdenum catalyst he could produce ammonia in a multistep reaction that can take place at room temperature and atmospheric pressure. The process is not yet efficient enough for industrial use, but Schrock hopes that with further refinement, it could be useful within the next 20 years.
“I don’t think it’s going to replace Haber-Bosch, but I think it can reduce the amount of carbon dioxide we release into the atmosphere,” he said.