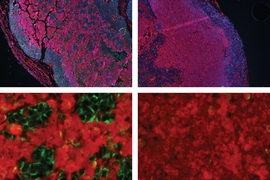
MIT biologists have discovered a fundamental mechanism that helps brain tumors called glioblastomas grow aggressively. After blocking this mechanism in mice, the researchers were able to halt tumor growth.
The researchers also identified a genetic marker that could be used to predict which patients would most likely benefit from this type of treatment. Glioblastoma is usually treated with radiation and the chemotherapy drug temozolamide, which may extend patients’ lifespans but in most cases do not offer a cure.
“There are very few specific or targeted inhibitors that are used in the treatment of brain cancer. There’s really a dire need for new therapies and new ideas,” says Michael Hemann, an associate professor of biology at MIT, a member of MIT’s Koch Institute for Integrative Cancer Research, and a senior author of the study.
Drugs that block a key protein involved in the newly discovered process already exist, and at least one is in clinical trials to treat cancer. However, most of these inhibitors do not cross the blood-brain barrier, which separates the brain from circulating blood and prevents large molecules from entering the brain. The MIT team hopes to develop drugs that can cross this barrier, possibly by packaging them into nanoparticles.
The study, which appears in Cancer Cell on Sept. 28, is a collaboration between the labs of Hemann; Jacqueline Lees, associate director of the Koch Institute and the Virginia and D.K. Ludwig Professor for Cancer Research; and Phillip Sharp, an MIT Institute Professor and member of the Koch Institute. The paper’s lead authors are former MIT postdoc Christian Braun, recent PhD recipient Monica Stanciu, and research scientist Paul Boutz.
Too much splicing
Several years ago, Stanciu and Braun came up with the idea to use a type of screen known as shRNA to seek genes involved in glioblastoma. This test involves using short strands of RNA to block the expression of specific genes. Using this approach, researchers can turn off thousands of different genes, one per tumor cell, and then measure the effects on cell survival.
One of the top hits from this screen was the gene for a protein called PRMT5. When this gene was turned off, tumor cells stopped growing. Previous studies had linked high levels of PRMT5 to cancer, but the protein is an enzyme that can act on hundreds of other proteins, so scientists weren’t sure exactly how it was stimulating cancer cell growth.
Further experiments in which the researchers analyzed other genes affected when PRMT5 was inhibited led them to hypothesize that PRMT5 was using a special type of gene splicing to stimulate tumor growth. Gene splicing is required to snip out portions of messenger RNA known as introns, that are not needed after the gene is copied into mRNA.
In 2015, Boutz and others in Sharp’s lab discovered that about 10 to 15 percent of human mRNA strands still have one to three “detained introns,” even though they are otherwise mature. Because of those introns, these mRNA molecules can’t leave the nucleus.
“What we think is that these strands are basically an mRNA reservoir. You have these unproductive isoforms sitting in the nucleus, and the only thing that keeps them from being translated is that one intron,” says Braun, who is now a physician-scientist at Ludwig Maximilian University of Munich.
In the new study, the researchers discovered that PRMT5 plays a key role in regulating this type of splicing. They speculate that neural stem cells utilize high levels of PRMT5 to guarantee efficient splicing and therefore expression of proliferation genes. “As the cells move toward their mature state, PRMT5 levels drop, detained intron levels rise, and those messenger RNAs associated with proliferation get stuck in the nucleus,” Lees says.
When brain cells become cancerous, PRMT5 levels are typically boosted and the splicing of proliferation-associated mRNA is improved, ultimately helping the cells to grow uncontrollably.
Predicting success
When the researchers blocked PRMT5 in tumor cells, they found that the cells stopped dividing and entered a dormant, nondividing state. PRMT5 inhibitors also halted growth of glioblastoma tumors implanted under the skin of mice, but they did not work as well in tumors located in the brain, because of the difficulties in crossing the blood-brain barrier.
Unlike many existing cancer treatments, the PRMT5 inhibitors did not appear to cause major side effects. The researchers believe this may be because mature cells are not as dependent as cancer cells on PRMT5 function.
The findings shed light on why researchers have previously found PRMT5 to be a promising potential target for cancer treatment, says Omar Abdel-Wahab, an assistant member in the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center, who was not involved in the study.
“PRMT5 has a lot of roles, and until now, it has not been clear what is the pathway that is really important for its contributions to cancer,” says Abdel-Wahab. “What they have found is that one of the key contributions is in this RNA splicing mechanism, and furthermore, when RNA splicing is disrupted, that key pathway is disabled.”
The researchers also discovered a biomarker that could help identify patients who would be most likely to benefit from a PRMT5 inhibitor. This marker is a ratio of two proteins that act as co-factors for PRMT5’s splicing activity, and reveals whether PRMT5 in those tumor cells is involved in splicing or some other cell function.
“This becomes really important when you think about clinical trials, because if 50 percent or 25 percent of tumors are going to have some response and the others are not, you may not have a way to target it toward those patients that may have a particular benefit. The overall success of the trial may be damaged by lack of understanding of who’s going to respond,” Hemann says.
The MIT team is now looking into the potential role of PRMT5 in other types of cancer, including lung tumors. They also hope to identify other genes and proteins involved in the splicing process they discovered, which could also make good drug targets.
Spearheaded by students and postdocs from several different labs, this project offers a prime example of the spirit of collaboration and “scientific entrepreneurship” found at MIT and the Koch Institute, the researchers say.
“I think it really is a classic example of how MIT is a sort of bottom-up place,” Lees says. “Students and postdocs get excited about different ideas, and they sit in on each other’s seminars and hear interesting things and pull them together. It really is an amazing example of the creativity that young people at MIT have. They’re fearless.”
The research was funded by the Ludwig Center for Molecular Oncology at MIT, the Koch Institute Frontier Research Program through the Kathy and Curt Marble Cancer Research Fund, the National Institutes of Health, and the Koch Institute Support (core) Grant from the National Cancer Institute.