Metal alloys such as steel and zirconium that are used in pipes for nuclear reactors and oil fields naturally acquire a protective oxide or sulfide layer. But hydrogen penetration can lead to their breakdown and speed up corrosion. Understanding how defects in the protective layer allow hydrogen to penetrate could lead to designing stronger, more corrosion resistant alloys.
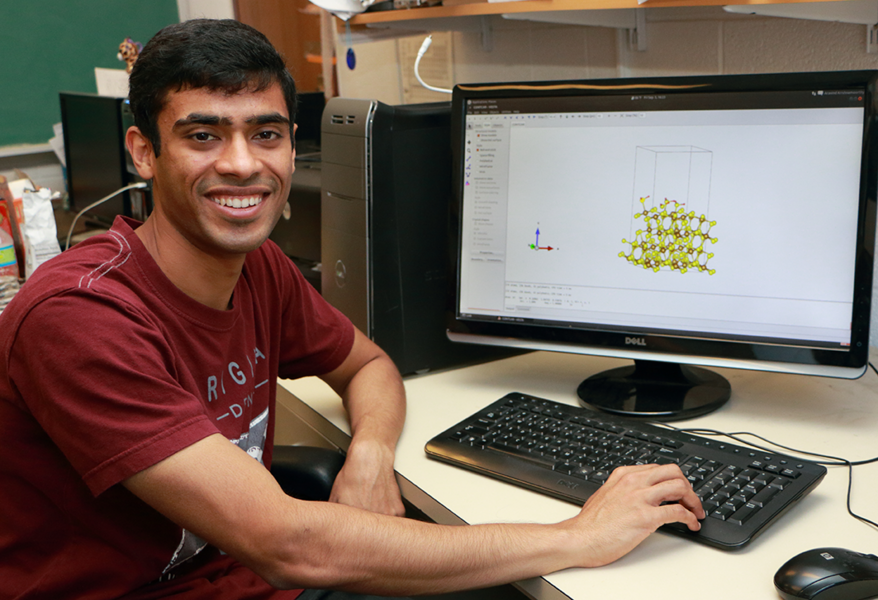
MIT postdoctoral associate Mostafa Youssef and graduate student Aravind Krishnamoorthy are studying protective layers of iron sulfide in steel and zirconium oxide in zirconium alloys by modeling atomic level interactions. They are members of the Lab for Electrochemical Interfaces, which is directed by MIT associate professor of nuclear science and engineering Bilge Yildiz.
"We are trying to first understand fundamentally this oxide layer that grows on the metal in general; it doesn't really matter which particular metal it is," Youssef says. "Then we can engineer through alloying by inserting a percentage of other metals, in order to improve the resistance toward the degradation phenomenon that we care about, whether it's hydrogen or just the continuous corrosion. We just need a little bit of corrosion to sufficiently produce a coherent oxide layer."
"The next level after the understanding would be engineering by suggesting alloying elements on a physics-based understanding rather than empirical experimentation," he adds. "What we are trying to show is that we can understand the physical mechanism by which the process takes place and then scientifically suggest a metal based on this new knowledge."
Lattice vacancies trap hydrogen
Youssef studied how point defects in zirconium oxide can move through the material from the environment side to the underlying metal. Zirconium oxide is the native protective layer that forms on zirconium alloys used in nuclear reactors, in particular for enclosing the nuclear fuel. "If this process goes very fast, then the corrosion reaction will proceed in a fast fashion as well, and continuously will eat the whole metal. We want corrosion to take place, but we don't want it to be very fast over long time scales. We want it to just provide a sufficient protective layer, and then stop," Youssef said.
"Hydrogen is dangerous because if it gets inside the metal, it degrades its mechanical properties completely, a phenomenon called hydrogen embrittlement, and leads to premature cracking, something clearly unwanted in a nuclear reactor," Youssef explains.
Youssef and Yildiz reported on hydrogen defects in zirconium oxide, under both oxygen rich/zirconium poor and oxygen poor/zirconium rich conditions, in the Physical Chemistry Chemical Physics article, "Hydrogen defects in tetragonal ZrO2 studied using density functional theory," in November 2013. Using Density Functional Theory calculations, they modeled both single and paired hydrogen atoms at different sites in the zirconium oxide crystal lattice and also modeled their charge states. Significantly, the research showed that both oxygen vacancies and zirconium vacancies could attract hydrogen under different conditions. The simulation found that "zirconium vacancies can act as trapping sites for hydrogen and the resulting complexes can be detrimental precursors for the mechanical failure of the oxide."
The work also included signatures of hydrogen in zirconia that could be experimentally validated, such as the net spin and the vibrational frequencies of the defects. Youssef is pursuing this theme by evaluating various compositions of zirconium alloys for their effectiveness at preventing hydrogen uptake through the zirconium oxide layer, which is a cause of premature fracture.
Modeling on atomic scale
While he also uses computational analysis, fifth-year materials science and engineering doctoral student Krishnamoorthy focuses on iron sulfide films inside pipes for offshore oil fields. He is studying how hydrogen gets into the native protective layer of iron sulfide, how mechanical damage propagates through the film, and whether there are ways to make it more stable. Lab member F. William Herbert, also a graduate student in materials science and engineering, handles the experimental side, studying formation and stability of iron sulfide on iron samples.
"One of the main strengths of our group is combining both the experimental data at the lab and the computational data that we provide to get a holistic picture of what's going on from the atomic scale," Krishnamoorthy says.
Krishnamoorthy worked with Herbert, Yildiz, and associate professor of materials science and engineering Krystyn J. Van Vliet on a paper that quantified the electronic band gap and surface states of iron sulfide, also known as pyrite, establishing significant differences between its surface properties and those of the bulk material. Krishnamoorthy contributed electronic structure calculations to the work, showing that the energy bandgap at the surface of iron sulfide is less than the comparable value for the bulk material. "This finding is important in establishing accurate models for predicting corrosion rates, and also for potential solar cell or semiconductor uses for the same material," Krishnamoorthy says.
On hydrogen-related research, "You have hydrogen that gets into the film, and it makes it mechanically weak, and then the film breaks off and the surface is no longer protected," Krishnamoorthy explains. "What you want to identify is what are the physical phenomena that are responsible for hydrogen entering the film and making it mechanically weak. We're trying to model them at the atomic scale, because that is the length scale at which the required reactions happen." He identified how atomic defects inside the iron sulfide films catalyze the hydrogen reactions and lead to mechanical fracture of the films. His recent work on this finding is in review prior to publication.
A challenge in this computational work is the scaling up from tiny atomic-level simulations that model very short timeframes to large, real-world models, in which corrosion happens over months or years. Youssef says, "If you think about zirconium alloys in a nuclear reactor, the industry has to produce structures that are several meters long, and for oil and gas field applications the structures could be kilometers of these alloys. What we are trying to do is just infer the fundamental physics underlying corrosion on a nanometer scale, and we are trying to transfer this understanding, which we obtained on the nanometer scale, to the engineering level, which takes place on the miles scale." Currently, Krishnamoorty is working on addressing this problem also by developing new models which bridge his atomistic simulations to large scales for corrosion prediction.
For atomistic simulations, they use commercially-available software for quantum-mechanical calculations. For example, Youssef used Vienna Ab-initio Simulation Package (VASP) software for his work on hydrogen defects in zirconium oxide. But they also write some of their own computer code for unique aspects of the passive films and crystal structures they study. Their investigations require a multi-disciplinary approach pulling in solid state physics, statistical mechanics, electrochemistry and materials science.
Krishnamoorthy received his bachelor's degree in metallurgical and materials engineering at the Indian Institute of Technology (IIT) in Madras, India. Youssef completed his master's and PhD at MIT in nuclear science and engineering. He received his bachelor's degree in nuclear engineering at Alexandria University in Egypt.