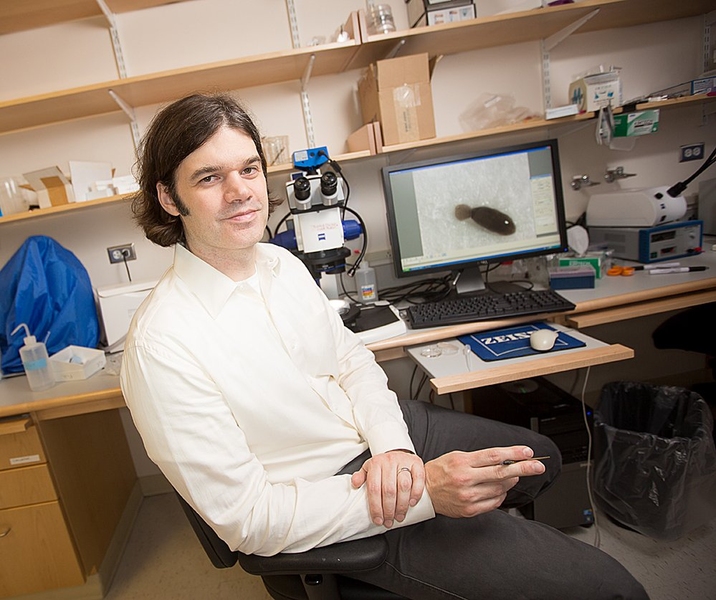
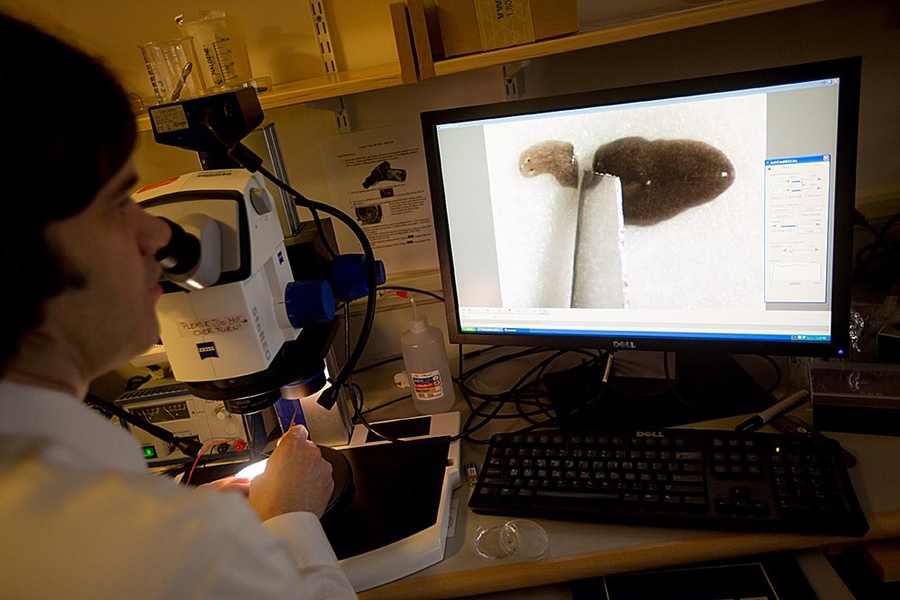
Few animals can rival the amazing regeneration abilities of the flatworms known as planarians: When the worms’ tails or heads are cut off, they grow new ones, and even a tiny piece of planarian tissue can regrow an entire animal.
Scientists first observed these phenomena more than a century ago, but until the past few years, they knew very little about how planarians achieve these incredible feats. MIT associate professor of biology Peter Reddien has made it his mission to discover the genetic and molecular basis of planarian regeneration, which he describes as one of the great mysteries of biology.
“Cellular and molecular insight into regeneration has come far in the past decade, but we’ve still got a long way to go to understand how an animal regrows a missing body part,” says Reddien, who is a core member of MIT’s Whitehead Institute. “That is the obsessive focus of my lab — to try to understand how regeneration happens, with the conviction that generation of fundamental knowledge about regeneration works will be important for understanding biology broadly and also for generating ideas for therapeutic applications.”
In recent years, Reddien’s lab has identified dozens of genes involved in planarian regeneration. Many of these are related to human genes, and some are active in response to human injuries. “It’s my hope that our continued work will enhance our understanding of what makes some animals great at regeneration and others not as good,” he says.
‘A golden era’
Growing up in Dallas, Reddien was drawn not to planarians but to planets. He closely followed the exploits of NASA, especially the travels of the Voyager spacecraft, with Voyager 2 reaching Neptune and heading out of the solar system by the time Reddien was 15 years old. “From a young age I thought I would be a physicist who would work for NASA or the Jet Propulsion Laboratory,” he says.
He entered the University of Texas as a physics major, but shifted gears after taking a required biology course.
“I realized that we were in a golden era for biological research, that this was going to be a period in history unlike any other for biological research … a period of great discoveries about how the fundamental attributes of life work,” Reddien recalls. “I found that exhilarating, and I got very excited about that as a future potential path for me.”
After graduating from college, Reddien came to MIT as a graduate student in molecular biology, working with Robert Horvitz, now the David H. Koch Professor of Biology. Among other projects, Horvitz’s lab was studying the molecular mechanisms of programmed cell death, a process critical to embryonic development and in defending against cancer.
Reddien finished his PhD in 2002 — the year Horvitz won the Nobel Prize in physiology or medicine for his work in programmed cell death — and went to the University of Utah to do postdoctoral research on regeneration. Reddien describes his decision at the time to launch into study of the molecular basis of regeneration in planarians as “a bit of a gamble.”
“There was a lot of potential, but it was off the radar and in its early stages as a molecular genetic field,” he says. “At that time, the tools for studying gene function in this organism were just in their infancy. There were no published roles for any gene at the time based on disrupting genes and studying what goes wrong in regeneration.”
At Utah, Reddien worked in the lab of Alejandro Sánchez Alvarado, who had recently shown that a new technique known as RNA interference, which allows genes to be selectively turned off, could work in planarians. Until that point, genetic studies of planarian regeneration had not been possible. Reddien was confident that new tools such as RNA interference could get planarians to reveal their regeneration secrets.
“No one had done it, and it was not an established system for taking that type of approach, so I did feel like I was taking a bit of a risk,” Reddien says. “It worked out better than I could have hoped, but I knew that the road was going to be full of challenges because there weren’t established paths to follow to study regeneration defects in these animals.”
A fundamental approach
Since joining the MIT faculty in 2005, Reddien has discovered dozens of genes that play key roles in regeneration, whether initiating the process or helping to determine which body part needs to be replaced. One gene that his lab investigated, known as notum, interacts with a cell-communication system called the Wnt signaling pathway to control whether an animal regrows a head or a tail.
Reddien also found that adult planarians maintain a population of pluripotent stem cells, known as clonogenic neoblasts, that can grow into any type of tissue. These cells are key to tissue regeneration, and his lab has identified genes that give these cells their regenerative potential.
“This is the kind of science you dream of when you’re a kid,” Reddien says. “We’re cutting off animals’ heads and figuring out how they regrow new ones at a molecular level. It’s up to us to develop the methods we need to solve these problems because it’s such a new field. It’s just been a real adventure and that’s something I’m greatly drawn to in science.”
Many of the genes that Reddien has discovered in planarians have counterparts in the human genome, though the functions of many in humans have been little studied. Learning more about them could help advance the field of regenerative medicine.
“We are taking a fundamental science approach to the problem, with the idea that evolution has already selected for mechanisms that allow regenerative repair events that would be the dream of regenerative medicine. The hope is that understanding these mechanisms could lead to new ideas about how applications could be derived to enhance wound healing and repair in humans,” Reddien says.
Scientists first observed these phenomena more than a century ago, but until the past few years, they knew very little about how planarians achieve these incredible feats. MIT associate professor of biology Peter Reddien has made it his mission to discover the genetic and molecular basis of planarian regeneration, which he describes as one of the great mysteries of biology.
“Cellular and molecular insight into regeneration has come far in the past decade, but we’ve still got a long way to go to understand how an animal regrows a missing body part,” says Reddien, who is a core member of MIT’s Whitehead Institute. “That is the obsessive focus of my lab — to try to understand how regeneration happens, with the conviction that generation of fundamental knowledge about regeneration works will be important for understanding biology broadly and also for generating ideas for therapeutic applications.”
In recent years, Reddien’s lab has identified dozens of genes involved in planarian regeneration. Many of these are related to human genes, and some are active in response to human injuries. “It’s my hope that our continued work will enhance our understanding of what makes some animals great at regeneration and others not as good,” he says.
‘A golden era’
Growing up in Dallas, Reddien was drawn not to planarians but to planets. He closely followed the exploits of NASA, especially the travels of the Voyager spacecraft, with Voyager 2 reaching Neptune and heading out of the solar system by the time Reddien was 15 years old. “From a young age I thought I would be a physicist who would work for NASA or the Jet Propulsion Laboratory,” he says.
He entered the University of Texas as a physics major, but shifted gears after taking a required biology course.
“I realized that we were in a golden era for biological research, that this was going to be a period in history unlike any other for biological research … a period of great discoveries about how the fundamental attributes of life work,” Reddien recalls. “I found that exhilarating, and I got very excited about that as a future potential path for me.”
After graduating from college, Reddien came to MIT as a graduate student in molecular biology, working with Robert Horvitz, now the David H. Koch Professor of Biology. Among other projects, Horvitz’s lab was studying the molecular mechanisms of programmed cell death, a process critical to embryonic development and in defending against cancer.
Reddien finished his PhD in 2002 — the year Horvitz won the Nobel Prize in physiology or medicine for his work in programmed cell death — and went to the University of Utah to do postdoctoral research on regeneration. Reddien describes his decision at the time to launch into study of the molecular basis of regeneration in planarians as “a bit of a gamble.”
“There was a lot of potential, but it was off the radar and in its early stages as a molecular genetic field,” he says. “At that time, the tools for studying gene function in this organism were just in their infancy. There were no published roles for any gene at the time based on disrupting genes and studying what goes wrong in regeneration.”
At Utah, Reddien worked in the lab of Alejandro Sánchez Alvarado, who had recently shown that a new technique known as RNA interference, which allows genes to be selectively turned off, could work in planarians. Until that point, genetic studies of planarian regeneration had not been possible. Reddien was confident that new tools such as RNA interference could get planarians to reveal their regeneration secrets.
“No one had done it, and it was not an established system for taking that type of approach, so I did feel like I was taking a bit of a risk,” Reddien says. “It worked out better than I could have hoped, but I knew that the road was going to be full of challenges because there weren’t established paths to follow to study regeneration defects in these animals.”
A fundamental approach
Since joining the MIT faculty in 2005, Reddien has discovered dozens of genes that play key roles in regeneration, whether initiating the process or helping to determine which body part needs to be replaced. One gene that his lab investigated, known as notum, interacts with a cell-communication system called the Wnt signaling pathway to control whether an animal regrows a head or a tail.
Reddien also found that adult planarians maintain a population of pluripotent stem cells, known as clonogenic neoblasts, that can grow into any type of tissue. These cells are key to tissue regeneration, and his lab has identified genes that give these cells their regenerative potential.
“This is the kind of science you dream of when you’re a kid,” Reddien says. “We’re cutting off animals’ heads and figuring out how they regrow new ones at a molecular level. It’s up to us to develop the methods we need to solve these problems because it’s such a new field. It’s just been a real adventure and that’s something I’m greatly drawn to in science.”
Many of the genes that Reddien has discovered in planarians have counterparts in the human genome, though the functions of many in humans have been little studied. Learning more about them could help advance the field of regenerative medicine.
“We are taking a fundamental science approach to the problem, with the idea that evolution has already selected for mechanisms that allow regenerative repair events that would be the dream of regenerative medicine. The hope is that understanding these mechanisms could lead to new ideas about how applications could be derived to enhance wound healing and repair in humans,” Reddien says.