Science and technology journalists pride themselves on the ability to explain complicated ideas in accessible ways, but there are some technical principles that we encounter so often in our reporting that paraphrasing them or writing around them begins to feel like missing a big part of the story. So in a new series of articles called "Explained," MIT News Office staff will explain some of the core ideas in the areas they cover, as reference points for future reporting on MIT research.
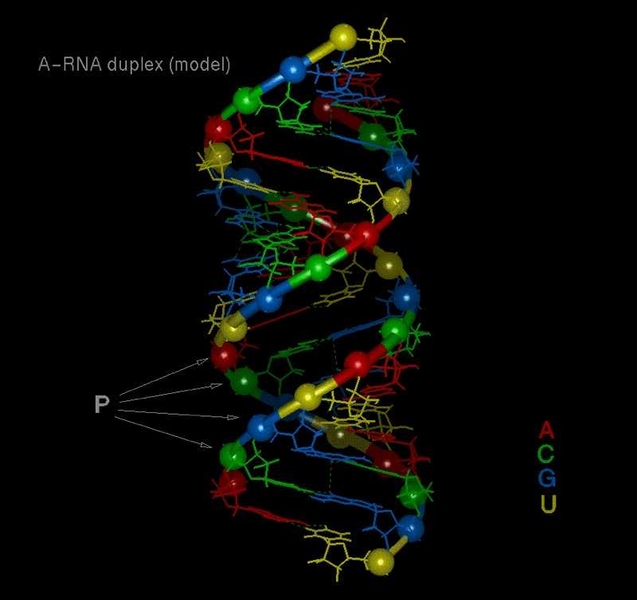
Every high school biology student learns the basics of how genes are expressed: DNA, the cell’s master information keeper, is copied into messenger RNA, which carries protein-building instructions to the ribosome, the part of the cell where proteins are assembled.
But it turns out the picture is far more complicated than that. In recent years, biologists have discovered a myriad of other molecules that fine-tune this process, including several types of RNA (ribonucleic acid). Through a naturally occurring phenomenon known as RNA interference, short strands of RNA can selectively intercept and destroy messenger RNA before it delivers its instructions.
Scientists are now pursuing disease treatments based on RNA interference (RNAi), which offers the tantalizing ability to shut down any gene in the body.
“With RNAi, we have the possibility to design small RNA that matches any gene, or any part of that gene, and silence it. Then we can ask what is the potential benefit of silencing that gene in the disease process,” says MIT Institute Professor Phillip Sharp, whose lab is pursuing such studies.
In 2006, the Nobel Prize in Physiology or Medicine was awarded to two scientists, including Andrew Fire, who earned his MIT PhD in 1983 under Sharp’s supervision, for the discovery of RNA interference. Fire and Craig Mello showed in 1998 that when short, double-stranded RNA molecules with sequences complementary to a specific messenger RNA were injected into the worm C. elegans, production of the protein encoded by that messenger RNA was halted.
Here’s how it works: Double-stranded RNA molecules called siRNA (short interfering RNA) bind to complementary messenger RNA, then enlist the help of proteins, the RNA-induced silencing complex. Those proteins cleave the chemical bonds holding messenger RNA together and prevent it from delivering its protein-building instructions.
This mechanism occurs naturally and may have evolved to give cells additional control over gene expression, particularly during embryonic development. It may also serve as a defense mechanism against viruses that try to insert their genetic material into cells.
RNA interference can also be mediated by microRNA, which is a short, single-stranded RNA molecule. RNA interference has been observed in a wide range of species, including plants, bacteria and fruit flies as well as humans.
Scientists have shown that synthetic siRNA injected into human cells in the lab can successfully shut off genes, raising hopes that diseases such as cancer, cystic fibrosis, Huntington’s disease and others caused by malfunctioning genes could be treated with RNA interference.
Before such therapies can become useful, scientists must figure out how to efficiently deliver small RNA molecules into target cells. Sharp and others at MIT, including Institute Professor Robert Langer and research scientist Daniel Anderson, are working on a delivery method that packages RNA inside a layer of fat-like molecules called lipidoids, which can cross cells’ fatty outer membrane. They have used the lipidoids to successfully deliver RNA to liver and lung cells in mice and monkeys, and hope to begin clinical trials within the next two years.
Sharp is also working with Sangeeta Bhatia, professor in the Harvard-MIT Division of Health Sciences and Technology, on better ways to target the RNA-carrying nanoparticles to specific cells, such as tumor cells.
There is a long way to go, says Sharp, but the potential of RNA interference is very large. “The discovery of RNA interference opened our eyes to a whole new aspect of biomedical science and biology that we just had never been aware of.”
Every high school biology student learns the basics of how genes are expressed: DNA, the cell’s master information keeper, is copied into messenger RNA, which carries protein-building instructions to the ribosome, the part of the cell where proteins are assembled.
But it turns out the picture is far more complicated than that. In recent years, biologists have discovered a myriad of other molecules that fine-tune this process, including several types of RNA (ribonucleic acid). Through a naturally occurring phenomenon known as RNA interference, short strands of RNA can selectively intercept and destroy messenger RNA before it delivers its instructions.
Scientists are now pursuing disease treatments based on RNA interference (RNAi), which offers the tantalizing ability to shut down any gene in the body.
“With RNAi, we have the possibility to design small RNA that matches any gene, or any part of that gene, and silence it. Then we can ask what is the potential benefit of silencing that gene in the disease process,” says MIT Institute Professor Phillip Sharp, whose lab is pursuing such studies.
In 2006, the Nobel Prize in Physiology or Medicine was awarded to two scientists, including Andrew Fire, who earned his MIT PhD in 1983 under Sharp’s supervision, for the discovery of RNA interference. Fire and Craig Mello showed in 1998 that when short, double-stranded RNA molecules with sequences complementary to a specific messenger RNA were injected into the worm C. elegans, production of the protein encoded by that messenger RNA was halted.
Here’s how it works: Double-stranded RNA molecules called siRNA (short interfering RNA) bind to complementary messenger RNA, then enlist the help of proteins, the RNA-induced silencing complex. Those proteins cleave the chemical bonds holding messenger RNA together and prevent it from delivering its protein-building instructions.
This mechanism occurs naturally and may have evolved to give cells additional control over gene expression, particularly during embryonic development. It may also serve as a defense mechanism against viruses that try to insert their genetic material into cells.
RNA interference can also be mediated by microRNA, which is a short, single-stranded RNA molecule. RNA interference has been observed in a wide range of species, including plants, bacteria and fruit flies as well as humans.
Scientists have shown that synthetic siRNA injected into human cells in the lab can successfully shut off genes, raising hopes that diseases such as cancer, cystic fibrosis, Huntington’s disease and others caused by malfunctioning genes could be treated with RNA interference.
Before such therapies can become useful, scientists must figure out how to efficiently deliver small RNA molecules into target cells. Sharp and others at MIT, including Institute Professor Robert Langer and research scientist Daniel Anderson, are working on a delivery method that packages RNA inside a layer of fat-like molecules called lipidoids, which can cross cells’ fatty outer membrane. They have used the lipidoids to successfully deliver RNA to liver and lung cells in mice and monkeys, and hope to begin clinical trials within the next two years.
Sharp is also working with Sangeeta Bhatia, professor in the Harvard-MIT Division of Health Sciences and Technology, on better ways to target the RNA-carrying nanoparticles to specific cells, such as tumor cells.
There is a long way to go, says Sharp, but the potential of RNA interference is very large. “The discovery of RNA interference opened our eyes to a whole new aspect of biomedical science and biology that we just had never been aware of.”