New research at MIT may help scientists better understand the chemical associations between chronic inflammation and diseases such as cancer and atherosclerosis. The work could lead to drugs that break the link between the two.
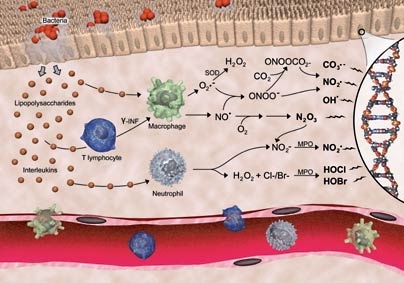
When an infection occurs, immune cells flock to the area and secrete large amounts of highly reactive chemicals to combat the invader. But, these inflammatory chemicals also attack normal tissue surrounding the infection and damage critical components of cells, including DNA. During chronic inflammation, that damage may lead to mutations or cell death and even to cancer and other diseases.
MIT researchers, led by toxicology graduate student Yelena Margolin of the Biological Engineering Division, have discovered that the DNA damage produced by one of these inflammatory chemicals, nitrosoperoxycarbonate, occurs at unexpected locations along the DNA helix. The finding counters the prevailing theory about where the DNA damage occurs and may shed light on new ways to diagnose and combat inflammation.
"We need to understand the mechanisms of inflammation in order to make new drugs that will break the link between inflammation and disease and to develop predictive biomarkers," said Dr. Peter Dedon, professor of toxicology and biological engineering and associate director of the Biological Engineering Division at MIT. "One of our goals is to develop biomarkers that can tell if you have inflammation and to define its extent, severity and location."
Margolin, Dedon and their colleagues at MIT and New York University reported their findings in a recent advance online issue of Nature Chemical Biology.
For years researchers have studied how the chemicals associated with the body's response to infection can damage DNA. That process begins with the removal of an electron from guanine, one of the four base building blocks that determine the genetic code in DNA. That removal is called oxidation, and guanine is the most easily oxidized of the four building blocks.
The prevailing theory has been that oxidation occurs most frequently when the guanine is sandwiched between two other guanine bases in the DNA helix.
By using comprehensive chemical screening and analysis of the frequency of DNA damage, the researchers found that a chemical produced during inflammation, nitrosoperoxycarbonate, actually caused oxidative damage at guanines that were supposed to be the least easily oxidized. The damage did not occur in clusters of guanine as expected, but rather at locations where guanine precedes cytosine, another of the four building blocks.
"That observation overturns the prevailing theory for predicting the location of DNA damage in the genome and complicates our understanding of the basis for diseases arising from chronic inflammation," said Dedon. "But it is likely to stir up discussions in the DNA damage and mutagenesis fields that could help us better understand the consequences of inflammation."
Margolin's and Dedon's colleagues on the paper are Jean-Francois Cloutier, auxiliary professor of pharmaceutical chemistry at Université Laval in Québec and formerly of the Dedon lab; Vladimir Shafirovich, research professor of chemistry at New York University; and Nicholas Geacintov, professor of chemistry and department chair at New York University.
The research was funded by the National Cancer Institute.