About 60 percent of all cancer patients in the United States receive radiation therapy as part of their treatment. However, this radiation can have severe side effects that often end up being too difficult for patients to tolerate.
Drawing inspiration from a tiny organism that can withstand huge amounts of radiation, researchers at MIT, Brigham and Women’s Hospital, and the University of Iowa have developed a new strategy that may protect patients from this kind of damage. Their approach makes use of a protein from tardigrades, often also called “water bears,” which are usually less than a millimeter in length.
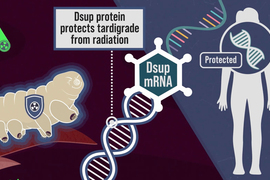
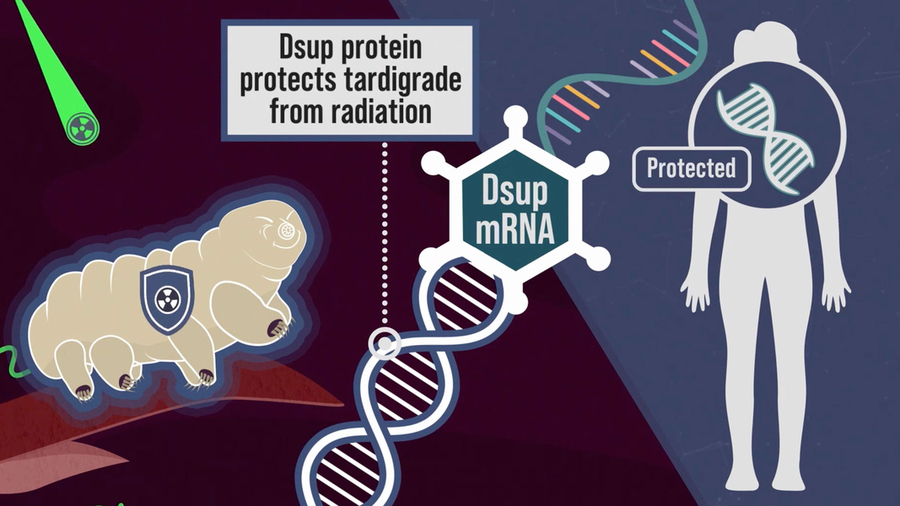
When the researchers injected messenger RNA encoding this protein into mice, they found that it generated enough protein to protect cells’ DNA from radiation-induced damage. If developed for use in humans, this approach could benefit many cancer patients, the researchers say.
“Radiation can be very helpful for many tumors, but we also recognize that the side effects can be limiting. There’s an unmet need with respect to helping patients mitigate the risk of damaging adjacent tissue,” says Giovanni Traverso, an associate professor of mechanical engineering at MIT and a gastroenterologist at Brigham and Women’s Hospital.
Traverso and James Byrne, an assistant professor of radiation oncology at the University of Iowa, are the senior authors of the study, which appears today in Nature Biomedical Engineering. The paper’s lead authors are Ameya Kirtane, an instructor in medicine at Harvard Medical School and a visiting scientist at MIT’s Koch Institute for Integrative Cancer Research, and Jianling Bi, a research scientist at the University of Iowa.
Extreme survival
Radiation is often used to treat cancers of the head and neck, where it can damage the mouth or throat, making it very painful to eat or drink. It is also commonly used for gastrointestinal cancers, which can lead to rectal bleeding. Many patients end up delaying treatments or stopping them altogether.
“This affects a huge number of patients, and it can manifest as something as simple as mouth sores, which can limit a person’s ability to eat because it’s so painful, to requiring hospitalization because people are suffering so terribly from the pain, weight loss, or bleeding. It can be pretty dangerous, and it’s something that we really wanted to try and address,” Byrne says.
Currently, there are very few ways to prevent radiation damage in cancer patients. There are a handful of drugs that can be given to try to reduce the damage, and for prostate cancer patients, a hydrogel can be used to create a physical barrier between the prostate and the rectum during radiation treatment.
For several years, Traverso and Byrne have been working on developing new ways to prevent radiation damage. In the new study, they were inspired by the extraordinary survival ability of tardigrades. Found all over the world, usually in aquatic environments, these organisms are well known for their resilience to extreme conditions. Scientists have even sent them into space, where they were shown to survive extreme dehydration and cosmic radiation.
One key component of tardigrades’ defense systems is a unique damage suppressor protein called Dsup, which binds to DNA and helps protect it from radiation-induced damage. This protein plays a major role in tardigrades’ ability to survive radiation doses 2,000 to 3,000 times higher than what a human being can tolerate.
When brainstorming ideas for novel ways to protect cancer patients from radiation, the researchers wondered if they might be able to deliver messenger RNA encoding Dsup to patient tissues before radiation treatment. This mRNA would trigger cells to transiently express the protein, protecting DNA during the treatment. After a few hours, the mRNA and protein would disappear.
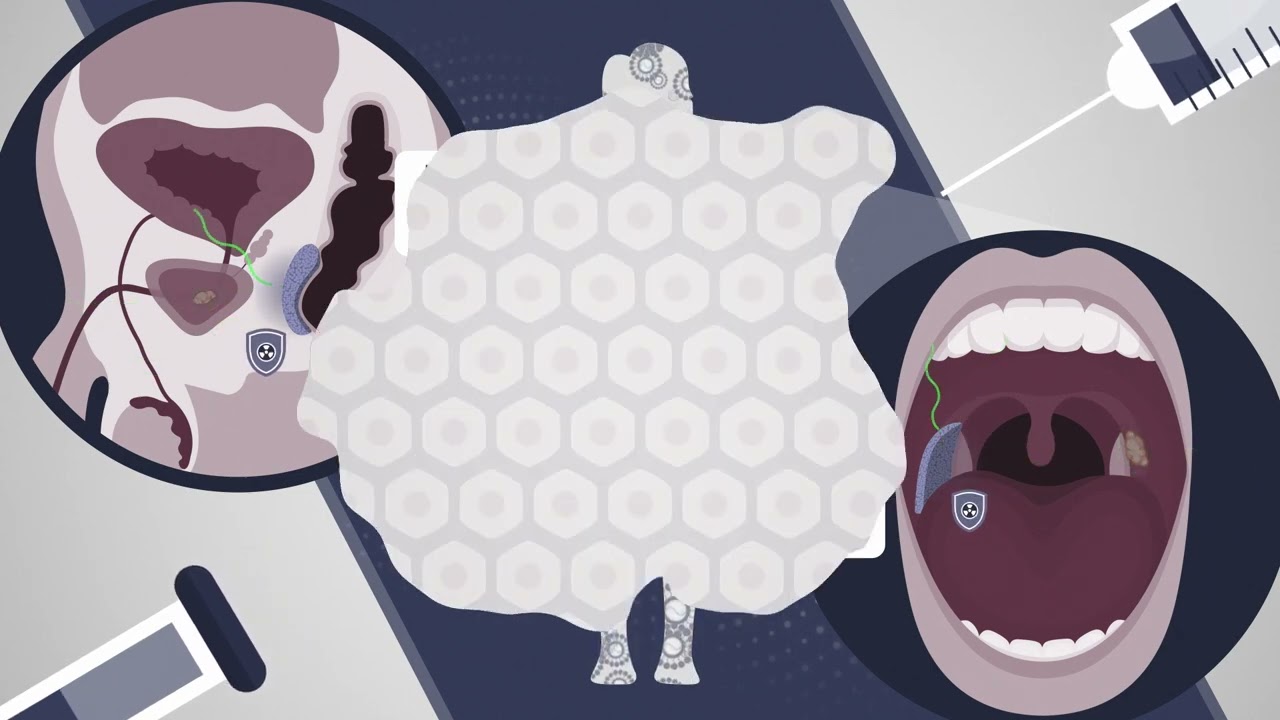
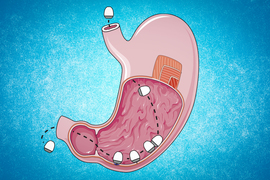
For this to work, the researchers needed a way to deliver mRNA that would generate large amounts of protein in the target tissues. They screened libraries of delivery particles containing both polymer and lipid components, which have been used separately to achieve efficient mRNA delivery. From these screens, they identified one polymer-lipid particle that was best-suited for delivery to the colon, and another that was optimized to deliver mRNA to mouth tissue.
“We thought that perhaps by combining these two systems — polymers and lipids — we may be able to get the best of both worlds and get highly potent RNA delivery. And that’s essentially what we saw,” Kirtane says. “One of the strengths of our approach is that we are using a messenger RNA, which just temporarily expresses the protein, so it’s considered far safer than something like DNA, which may be incorporated into the cells’ genome.”
Protection from radiation
After showing that these particles could successfully deliver mRNA to cells grown in the lab, the researchers tested whether this approach could effectively protect tissue from radiation in a mouse model.
They injected the particles into either the cheek or the rectum several hours before giving a dose of radiation similar to what cancer patients would receive. In these mice, the researchers saw a 50 percent reduction in the amount of double-stranded DNA breaks caused by radiation.
“This study shows great promise and is a really novel idea leveraging natural mechanisms of protection again DNA damage for the purpose of protecting healthy cells during radiation treatments for cancer,” says Ben Ho Park, director of the Vanderbilt-Ingram Cancer Center at Vanderbilt University Medical Center, who was not involved in the study.
The researchers also showed that the protective effect of the Dsup protein did not spread beyond the injection site, which is important because they don’t want to protect the tumor itself from the effects of radiation. To make this treatment more feasible for potential use in humans, the researchers now plan to work on developing a version of the Dsup protein that would not provoke an immune response, as the original tardigrade protein likely would.
If developed for use in humans, this protein could also potentially be used to protect against DNA damage caused by chemotherapy drugs, the researchers say. Another possible application would be to help prevent radiation damage in astronauts in space.
Other authors of the paper include Netra Rajesh, Chaoyang Tang, Miguel Jimenez, Emily Witt, Megan McGovern, Arielle Cafi, Samual Hatfield, Lauren Rosenstock, Sarah Becker, Nicole Machado, Veena Venkatachalam, Dylan Freitas, Xisha Huang, Alvin Chan, Aaron Lopes, Hyunjoon Kim, Nayoon Kim, Joy Collins, Michelle Howard, Srija Manchkanti, and Theodore Hong.
The research was funded by the Prostate Cancer Foundation Young Investigator Award, the U.S. Department of Defense Prostate Cancer Program Early Investigator Award, a Hope Funds for Cancer Research Fellowship, the American Cancer Society, the National Cancer Institute, MIT’s Department of Mechanical Engineering, and the U.S. Advanced Research Projects Agency for Health.