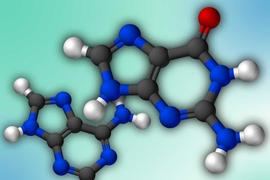
DNA is made from four nucleosides, each known by its own letter — A, G, C, and T. However, since the structure of DNA was deciphered in 1953, scientists have discovered several other variants that are often added to the DNA sequences to replace one of the usual four letters.
These variants, which may be modified versions of the traditional nucleosides, often help cells to control which genes are turned on and off, and are referred to as “epigenetic marks” in the DNA. In bacteria, they can also protect DNA from invasion by other organisms such as viruses.
Until now, these DNA modifications have been discovered by chance, as scientists uncovered unexpected signals in chemical analyses of DNA. However, a new approach from MIT, the University of Florida, and other institutions offers a systematic approach to discovering unknown epigenetic marks and modifications.
“It’s a way to discover nucleic acid modifications that you didn’t know existed,” says Peter Dedon, the Underwood-Prescott Professor of Biological Engineering at MIT. “We’ve developed a technology platform for the discovery and characterization of these new modifications.”
Dedon and his colleagues suspect that bacteria and viruses, in particular, have many DNA modifications that have not been discovered yet, which could offer new antibiotic targets and new tools for biotechnology. Using their approach, which combines bioanalytical chemistry, comparative genomics, and a special type of DNA sequencing, the team has discovered a DNA modification that helps bacteria to protect their genomes from viral infection. They report the findings in the Proceedings of the National Academy of Sciences the week of Feb. 29.
A multipronged approach
DNA modifications are usually inserted by enzymes into DNA after it is synthesized during cell division. Such modifications are often used as markers that help tell a cell which genes should be turned on at a given time. The DNA modifications can also help cells defend their DNA from attack by foreign DNA from viruses and other bacteria. A larger variety of similar modifications also help all types of RNA, including messenger RNA and transfer RNA, perform their functions.
Dedon and Valérie de Crécy-Lagard, a professor of microbiology and cell science at the University of Florida, decided to take a more systematic approach to discovering such modifications.
De Crécy-Lagard had previously discovered many of the genes required for the synthesis of RNA modifications known as queuosine and archeosine, which are found in microorganisms and are derived from a common precursor called preQ0. Using comparative genomics, a technique for screening the genomes of different organisms for variants of specific DNA sequences, she found similar genes in multiple bacterial species, located in a specific gene cluster that contained genes for DNA modification. Comparative genomics, one leg of the platform, thus provided the first clue about a potential new DNA modification.
De Crécy-Lagard and Dedon, both senior authors of the new PNAS paper, decided to test de Crécy-Lagard’s prediction that these bacteria insert preQ0 into DNA. Using mass spectrometry, which allows researchers to both search for unknown molecules and find those with a specific mass, Dedon’s lab found DNA modified with preQ0-like structures, which the team named dADG, in bacteria with the modification gene cluster but not in bacteria that lacked it.
The researchers then showed that, in the types of bacteria they studied, the dADG modification is part of a defense system that protects the bacterial cell DNA. These bacteria produce enzymes called restriction enzymes that chop up the unmodified DNA of an invading virus, for example. Dedon and de Crécy-Lagard are now searching in other bacteria for different epigenetic functions for these DNA modifications.
In a collaboration with Richard Roberts, chief scientific officer at New England Biolabs, the team is now applying the third element of their technology platform to characterize the new dADG modification — a special kind of DNA sequencing called single-molecule real-time sequencing (SMRT), done on machines made by Pacific Biosciences. SMRT sequencing often picks up a signal when it encounters something other than the traditional four nucleosides.
“There’s a little hiccup in the process, a tiny little pause, and if you tune the software correctly, you can pick up that signal and know where it is in the genome,” Dedon says. “This lets you map DNA modifications across the genome.” This type of sequencing doesn’t reveal what the modification is, but it pinpoints its location.
By combining the observational power of SMRT sequencing with the predictive power of comparative genomics and the ability to detect and identify modifications using bioanalytics, scientists can find a new modification, figure out what the modification is, and discover its role.
“This paper continues a remarkable series of investigations from the Dedon laboratory concerning novel DNA chemistry,” says Christopher Mathews, a professor emeritus of biochemistry and biophysics at Oregon State University, who was not involved in the research. “Phylogenetic analysis suggests that the genes for some of the novel synthetic pathways represent the basis for hitherto unknown restriction-modification systems.”
Antibiotic targets
In humans, there are only a few known DNA modifications, most of which weren’t discovered until decades after the four traditional DNA bases were identified. “Are there more out there? It’s an interesting question,” Dedon says. “There probably aren’t a huge number, but we could go look for more in humans.”
In bacteria, on the other hand, the team believes there may be at least a dozen more modifications that haven’t been found yet, and even more in bacteriophages (viruses that infect bacteria). One of the important connections between bacterial DNA modifications and humans lies in the bacteria of the human gut — the gut microbiome. The team now has evidence that bacteria in the gut microbiome contain dADG, as well as another bacterial DNA modification that they discovered called phosphorothioates, and they are looking to see if they play any role in human health and disease.
Any new modifications discovered could also become useful antibiotic targets, especially those that prevent restriction enzymes from chopping up the bacteria’s own DNA. Drugs that inhibit the enzyme that inserts the DNA modification would disrupt the defense system, allowing the restriction enzyme to destroy the host cell’s DNA. In some bacteria, the modifications may also be essential as epigenetic marks and thus antibiotic targets as well.
“A lot of these enzymes are unique to the bacterium and they’re also essential to the survival of that organism, so they might make good antibiotic targets,” Dedon says.
The research was funded by the Singapore-MIT Alliance for Research and Technology (SMART) and the National Research Foundation Singapore under the Campus for Research Excellence and Technological Enterprise (CREATE) program.