For many applications such as biomedical, mechanical, or environmental monitoring devices, harnessing the energy of small motions could provide a small but virtually unlimited power supply. While a number of approaches have been attempted, researchers at MIT have now developed a completely new method based on electrochemical principles, which could be capable of harvesting energy from a broader range of natural motions and activities, including walking.
The new system, based on the slight bending of a sandwich of metal and polymer sheets, is described in the journal Nature Communications, in a paper by MIT professor Ju Li, graduate students Sangtae Kim and Soon Ju Choi, and four others.
Most previously designed devices for harnessing small motions have been based on the triboelectric effect (essentially friction, like rubbing a balloon against a wool sweater) or piezoelectrics (crystals that produce a small voltage when bent or compressed). These work well for high-frequency sources of motion such as those produced by the vibrations of machinery. But for typical human-scale motions such as walking or exercising, such systems have limits.
“When you put in an impulse” to such traditional materials, “they respond very well, in microseconds. But this doesn’t match the timescale of most human activities,” says Li, who is the Battelle Energy Alliance Professor in Nuclear Science and Engineering and professor of materials science and engineering. “Also, these devices have high electrical impedance and bending rigidity and can be quite expensive,” he says.
Simple and flexible
By contrast, the new system uses technology similar to that in lithium ion batteries, so it could likely be produced inexpensively at large scale, Li says. In addition, these devices would be inherently flexible, making them more compatible with wearable technology and less likely to break under mechanical stress.
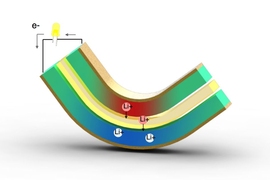
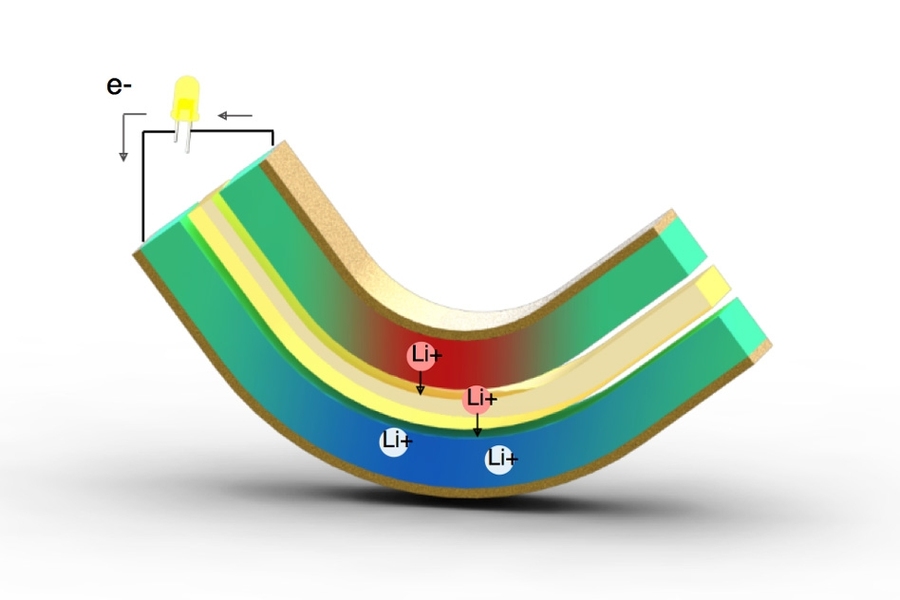
While piezoelectric materials are based on a purely physical process, the new system is electrochemical, like a battery or a fuel cell. It uses two thin sheets of lithium alloys as electrodes, separated by a layer of porous polymer soaked with liquid electrolyte that is efficient at transporting lithium ions between the metal plates. But unlike a rechargeable battery, which takes in electricity, stores it, and then releases it, this system takes in mechanical energy and puts out electricity.
When bent even a slight amount, the layered composite produces a pressure difference that squeezes lithium ions through the polymer (like the reverse osmosis process used in water desalination). It also produces a counteracting voltage and an electrical current in the external circuit between the two electrodes, which can be then used directly to power other devices.
Because it requires only a small amount of bending to produce a voltage, such a device could simply have a tiny weight attached to one end to cause the metal to bend as a result of ordinary movements, when strapped to an arm or leg during everyday activities. Unlike batteries and solar cells, the output from the new system comes in the form of alternating current (AC), with the flow moving first in one direction and then the other as the material bends first one way and then back.
This device converts mechanical to electrical energy; therefore, “it is not limited by the second law of thermodynamics,” Li says, which sets an upper limit on the theoretically possible efficiency. “So in principle, [the efficiency] could be 100 percent,” he says. In this first-generation device developed to demonstrate the electrochemomechanical working principle, he says, “the best we can hope for is about 15 percent” efficiency. But the system could easily be manufactured in any desired size and is amenable to industrial manufacturing process.
Test of time
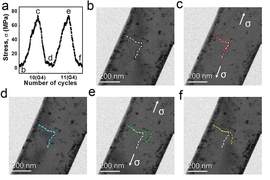
The test devices maintain their properties through many cycles of bending and unbending, Li reports, with little reduction in performance after 1,500 cycles. “It’s a very stable system,” he says.
Previously, the phenomenon underlying the new device “was considered a parasitic effect in the battery community,” according to Li, and voltage put into the battery could sometimes induce bending. “We do just the opposite,” Li says, putting in the stress and getting a voltage as output. Besides being a potential energy source, he says, this could also be a complementary diagnostic tool in electrochemistry. “It’s a good way to evaluate damage mechanisms in batteries, a way to understand battery materials better,” he says.
In addition to harnessing daily motion to power wearable devices, the new system might also be useful as an actuator with biomedical applications, or used for embedded stress sensors in settings such as roads, bridges, keyboards, or other structures, the researchers suggest.
“This work is very interesting and significant in the sense that it provides a novel approach to converting mechanical energy through an electrochemical route, using a simple design and device structure,” says Wu Wenzhuo, an assistant professor of industrial engineering at Purdue University who was not involved in this work. “More significantly, the output current from the demonstrated device is very large, with a long pulse duration. This is very important for practical applications, since most other mechanical energy harvesting methods suffer from the issues of small current output with short pulse duration.”
Wenzhuo adds that “efficient harvesting of such mechanical energies will help to develop more capable and intelligent wearable devices and human-machine interfaces. … This work presents huge potential in many applications such as flexible electronics, self-powered sensors, wearable devices, human-machine interfaces, robotics, artificial skin, etc.”
The team also included postdoc Kejie Zhao (now assistant professor at Purdue University) and visiting graduate student Giorgia Gobbi , and Hui Yang and Sulin Zhang at Penn State. The work was supported by the National Science Foundation, the MIT MADMEC Contest, the Samsung Scholarship Foundation, and the Kwanjeong Educational Foundation.