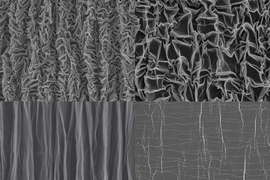
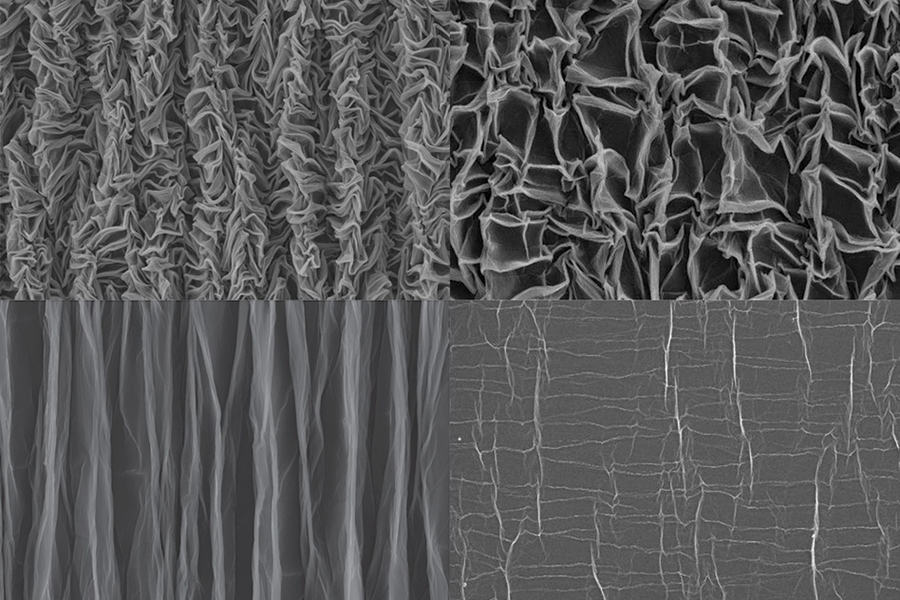
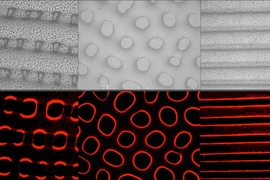
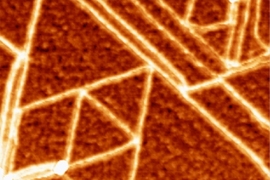
When someone crumples a sheet of paper, that usually means it’s about to be thrown away. But researchers have now found that crumpling a piece of graphene “paper” — a material formed by bonding together layers of the two-dimensional form of carbon — can actually yield new properties that could be useful for creating extremely stretchable supercapacitors to store energy for flexible electronic devices.
The finding is reported in the journal Scientific Reports by MIT’s Xuanhe Zhao, the Brit (1961) and Alex (1949) d'Arbeloff Career Development Associate Professor in Engineering Design, and four other authors. The new, flexible supercapacitors should be easy and inexpensive to fabricate, the team says.
“Many people are exploring graphene paper: It’s a good candidate for making supercapacitors, because of its large surface area per mass,” Zhao says. Now, he says, the development of flexible electronic devices, such as wearable or implantable biomedical sensors or monitoring devices, will require flexible power-storage systems.
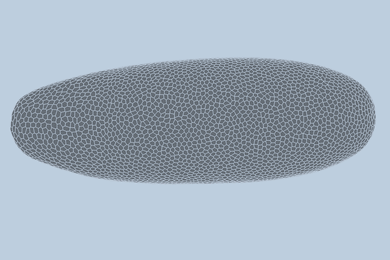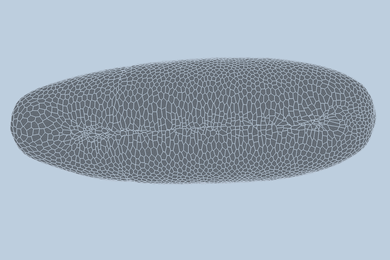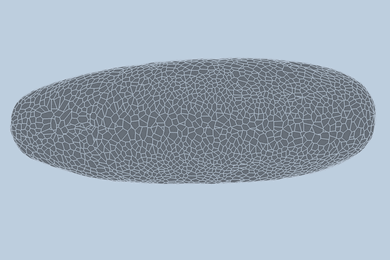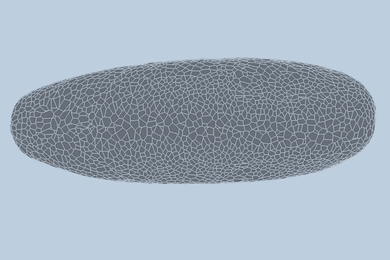
Like batteries, supercapacitors can store electrical energy, but they primarily do so electrostatically, rather than chemically — meaning they can deliver their energy faster than batteries can. Now Zhao and his team have demonstrated that by crumpling a sheet of graphene paper into a chaotic mass of folds, they can make a supercapacitor that can easily be bent, folded, or stretched to as much as 800 percent of its original size. The team has made a simple supercapacitor using this method as a proof of principle.
The material can be crumpled and flattened up to 1,000 times, the team has demonstrated, without a significant loss of performance. “The graphene paper is pretty robust,” Zhao says, “and we can achieve very large deformations over multiple cycles.” Graphene, a structure of pure carbon just one atom thick with its carbon atoms arranged in a hexagonal array, is one of the strongest materials known.
To make the crumpled graphene paper, a sheet of the material was placed in a mechanical device that first compressed it in one direction, creating a series of parallel folds or pleats, and then in the other direction, leading to a chaotic, rumpled surface. When stretched, the material’s folds simply smooth themselves out.
Forming a capacitor requires two conductive layers — in this case, two sheets of crumpled graphene paper — with an insulating layer in between, which in this demonstration was made from a hydrogel material. Like the crumpled graphene, the hydrogel is highly deformable and stretchable, so the three layers remain in contact even while being flexed and pulled.
Though this initial demonstration was specifically to make a supercapacitor, the same crumpling technique could be applied to other uses, Zhao says. For example, the crumpled graphene material might be used as one electrode in a flexible battery, or could be used to make a stretchable sensor for specific chemical or biological molecules.
“This work is really exciting and amazing to me,” says Dan Li, a professor of materials engineering at Monash University in Australia who was not involved in this research. He says the team “provides an extremely simple but highly effective concept to make stretchable electrodes for supercapacitors by controlled crumpling of multilayered graphene films.” While other groups have made flexible supercapacitors, he says, “Making supercapacitors stretchable has been a great challenge. This paper provides a very smart way to tackle this challenge, which I believe will bring wearable energy storage devices closer.”
The research team also included Jianfeng Zang at Huazhong University of Science and Technology and Changyang Cao, Yaying Feng, and Jie Liu at Duke University. The work was supported by the Office of Naval Research, the National Science Foundation, and the National 1000 Talents Program of China.