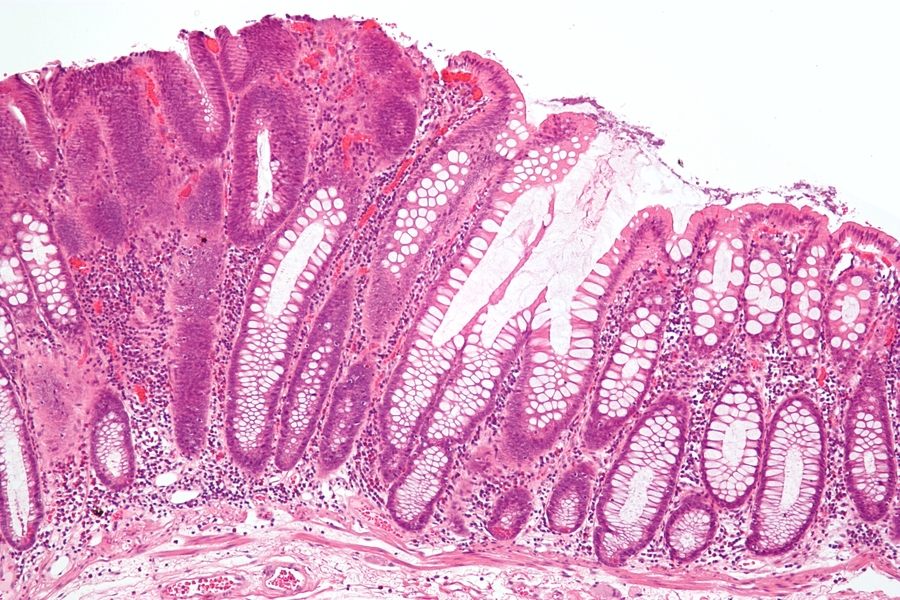
A major risk factor for colon cancer — the second leading cause of cancer death in the United States — is chronic inflammation of the colon. Nearly 10 percent of patients with inflammatory bowel disease (IBD) eventually develop colon cancer.
A new study from MIT demonstrates that this cancer arises from a specific type of DNA damage produced during inflammation. The researchers, led by Leona Samson, professor of biological engineering and biology, showed that mice that cannot repair this type of DNA damage are susceptible to colon cancer.
The team also identified three enzymes critical to repairing this damage. Previous studies have shown that humans produce widely varying amounts of one of the enzymes, so the findings offer a possible explanation for why some IBD patients are more likely to develop colon cancer, Samson says. Measuring these enzyme levels in a particular patient could help predict that patient’s risk of colon cancer.
“All other things being equal, if the same inflammatory response is present in the colon of different individuals, but they have differences in DNA-repair capability, they’re probably going to respond differently,” says Samson, who also a member of MIT’s Center for Environmental Health Sciences.
Jennifer Calvo, a research scientist in Samson’s lab, and Lisiane Meira, a former MIT scientist now at the University of Surrey, are lead authors of a paper on the new work appearing in the June 11 online edition of the Journal of Clinical Investigation.
Repair failure
During the chronic inflammation seen in conditions such as IBD (which includes Crohn’s disease and ulcerative colitis), immune cells release highly destructive chemicals known as reactive oxygen and nitrogen species. These chemicals are meant to kill bacteria and other invading pathogens, but if the chemicals linger too long, they can damage DNA and other molecules in healthy cells.
Four years ago, Samson’s lab showed that these reactive molecules produce a specific type of DNA damage that leads to colon cancer in mice. In that study, the researchers found that a DNA repair enzyme called AAG was necessary to repair this type of damage, known as etheno-base lesions. Mice lacking the AAG enzyme were much more susceptible to inflammation-induced colon cancer.
In the new study, funded by the National Institutes of Health, Samson and her colleagues looked at the effects of two additional DNA-repair enzymes. These enzymes, known as ALKBH2 and ALKBH3, were previously shown to be involved in repairing etheno-base lesions.
To induce colon inflammation in mice, researchers treat them with a chemical called dextran sodium sulfate (DSS), which destroys the mucosal lining of the intestines, allowing bacteria to reach the epithelial cells and provoke an inflammatory response. Following such inflammation, mice missing both ALKBH2 and ALKBH3 were much more susceptible to colon cancer than normal mice; the results were even more dire among mice missing all three DNA repair enzymes.
After analyzing tissue samples, the researchers discovered that all of the mice had initially experienced similar extensive colon damage, but mice with at least one of the three DNA repair enzymes were able to repair most or all of the tissue damage. Mice lacking all three enzymes couldn’t perform any tissue repair whatsoever.
Achieving DNA repair is especially critical in stem cells required to regenerate tissue. “Whatever cells are required for this tissue reconstruction are presumably being killed off because they can’t repair the DNA damage induced during inflammation,” Samson says.
The exact nature of the interactions between the three enzymes is unknown, but Samson speculates that AAG may first bind to the DNA lesions and then recruit ALKBH2 and ALKBH3 to help repair them.
Steven Lipkin, an associate professor of medicine at Weill Cornell Medical College, says the research “elegantly demonstrates” that distinct DNA-repair pathways are involved in repairing DNA damage caused by inflammation in the colon.
“This study gives insights that will help us to understand the precise mechanisms that cause inflammatory bowel disease-associated colorectal cancer, and will open the door to discovering better targeted chemopreventative agents,” says Lipkin, who was not involved in this study.
The MIT team is now looking into how varying levels of the AAG enzyme affect overall health, and is also planning to study how all three of these DNA-repair enzymes respond to inflammation induced by Helicobacter pylori infection, which can lead to stomach cancer.
A new study from MIT demonstrates that this cancer arises from a specific type of DNA damage produced during inflammation. The researchers, led by Leona Samson, professor of biological engineering and biology, showed that mice that cannot repair this type of DNA damage are susceptible to colon cancer.
The team also identified three enzymes critical to repairing this damage. Previous studies have shown that humans produce widely varying amounts of one of the enzymes, so the findings offer a possible explanation for why some IBD patients are more likely to develop colon cancer, Samson says. Measuring these enzyme levels in a particular patient could help predict that patient’s risk of colon cancer.
“All other things being equal, if the same inflammatory response is present in the colon of different individuals, but they have differences in DNA-repair capability, they’re probably going to respond differently,” says Samson, who also a member of MIT’s Center for Environmental Health Sciences.
Jennifer Calvo, a research scientist in Samson’s lab, and Lisiane Meira, a former MIT scientist now at the University of Surrey, are lead authors of a paper on the new work appearing in the June 11 online edition of the Journal of Clinical Investigation.
Repair failure
During the chronic inflammation seen in conditions such as IBD (which includes Crohn’s disease and ulcerative colitis), immune cells release highly destructive chemicals known as reactive oxygen and nitrogen species. These chemicals are meant to kill bacteria and other invading pathogens, but if the chemicals linger too long, they can damage DNA and other molecules in healthy cells.
Four years ago, Samson’s lab showed that these reactive molecules produce a specific type of DNA damage that leads to colon cancer in mice. In that study, the researchers found that a DNA repair enzyme called AAG was necessary to repair this type of damage, known as etheno-base lesions. Mice lacking the AAG enzyme were much more susceptible to inflammation-induced colon cancer.
In the new study, funded by the National Institutes of Health, Samson and her colleagues looked at the effects of two additional DNA-repair enzymes. These enzymes, known as ALKBH2 and ALKBH3, were previously shown to be involved in repairing etheno-base lesions.
To induce colon inflammation in mice, researchers treat them with a chemical called dextran sodium sulfate (DSS), which destroys the mucosal lining of the intestines, allowing bacteria to reach the epithelial cells and provoke an inflammatory response. Following such inflammation, mice missing both ALKBH2 and ALKBH3 were much more susceptible to colon cancer than normal mice; the results were even more dire among mice missing all three DNA repair enzymes.
After analyzing tissue samples, the researchers discovered that all of the mice had initially experienced similar extensive colon damage, but mice with at least one of the three DNA repair enzymes were able to repair most or all of the tissue damage. Mice lacking all three enzymes couldn’t perform any tissue repair whatsoever.
Achieving DNA repair is especially critical in stem cells required to regenerate tissue. “Whatever cells are required for this tissue reconstruction are presumably being killed off because they can’t repair the DNA damage induced during inflammation,” Samson says.
The exact nature of the interactions between the three enzymes is unknown, but Samson speculates that AAG may first bind to the DNA lesions and then recruit ALKBH2 and ALKBH3 to help repair them.
Steven Lipkin, an associate professor of medicine at Weill Cornell Medical College, says the research “elegantly demonstrates” that distinct DNA-repair pathways are involved in repairing DNA damage caused by inflammation in the colon.
“This study gives insights that will help us to understand the precise mechanisms that cause inflammatory bowel disease-associated colorectal cancer, and will open the door to discovering better targeted chemopreventative agents,” says Lipkin, who was not involved in this study.
The MIT team is now looking into how varying levels of the AAG enzyme affect overall health, and is also planning to study how all three of these DNA-repair enzymes respond to inflammation induced by Helicobacter pylori infection, which can lead to stomach cancer.