Scientists have long sought the ability to regenerate nerve cells, or neurons, which could offer a new way to treat spinal-cord damage as well as neurological diseases such as Alzheimer’s or Parkinson’s. Many chemicals can regenerate neurons grown in Petri dishes in the lab, but it’s difficult and time-consuming to identify those chemicals that work in live animals, which is critical for developing drugs for humans.
Engineers at MIT have now used a new microchip technology to rapidly test potential drugs on tiny worms called C. elegans, which are often used in studies of the nervous system. Using the new technology, associate professor Mehmet Fatih Yanik and his colleagues rapidly performed laser surgery, delivered drugs and imaged the resulting neuron regrowth in thousands of live animals.
“Our technology helps researchers rapidly identify promising chemicals that can then be tested in mammals and perhaps even in humans,” says Yanik. Using this technique, the researchers have already identified one promising class of neuronal regenerators.
The paper will appear in the online edition of the Proceedings of the National Academy of Sciences the week of Oct. 11.
Lead authors of the paper are postdoctoral associate Chrysanthi Samara and graduate students Christopher Rohde and Cody Gilleland, and collaborating chemists are Steve Haggarty and Stephanie Norton. Development of the new technology and the regeneration screen was funded by the NIH Director’s New Innovator Award Program, a Packard Fellowship in Science and Engineering, an Alfred Sloan Award in Neuroscience, an NSF Graduate Fellowship and a Merck Graduate Fellowship.
Rapid analysis
C. elegans is a useful model organism for neuron regeneration because it is optically transparent, and its entire neural network is known. Yanik and colleagues had previously developed a femtosecond laser nanosurgery technique which allowed them to cut and observe regeneration of individual axons — long extensions of neurons that send signals to neighboring cells. Their femtosecond laser nanosurgery technique uses tightly-focused infrared laser pulses that are shorter than billionth of a second. This allows the laser to penetrate deep into the animals without damaging the tissues on its way, until the laser beam hits its final target.
In the PNAS study, the researchers used their microchip technology to rapidly cut the axons of single neurons that sense touch. Moving single worms from their incubation well to an imaging microchip, immobilizing them and performing laser surgery takes only about 20 seconds, which allows thousands of surgeries to be performed in a short period of time.
After laser surgery, each worm is returned to its incubation well and treated with a different chemical compound. C. elegans neurons can partially regrow without help, which allowed Yanik’s team to look for drugs that can either enhance or inhibit this regrowth. After two or three days, the researchers imaged each worm to see if the drugs had any effect.
The MIT team found that a compound called staurosporine, which inhibits certain enzymes known as PKC kinases, had the strongest inhibitory effect. In a follow-up study, they tested some compounds that activate these kinases, and found that one of them stimulated regeneration of neurons significantly. Some of Yanik’s students are now testing those compounds on neurons derived from human embryonic stem cells.
The new technology represents a significant advance in the level of automation that can be achieved in C. elegans studies, says Michael Bastiani, professor of biology at the University of Utah. “Using ‘classical’ handling techniques you can cut and assay at most 100 animals per day,” he says. “Yanik's automated system seems like it could increase throughput by at least 10-fold over that number.” He points out that one potential limitation of the system is that it might not pick up the effects of neural regenerators that can’t penetrate the worm’s cuticle, a thick outer layer that surrounds the skin.
However, chemicals can still be taken up through the worms’ digestive tract, which is an important test for checking whether chemicals would work on live animals, says Yanik.
This microchip technology can also be used to screen compounds for their effects on other diseases such as Alzheimer’s, Parkinson’s and ALS, says Yanik.
Microchip technology rapidly identifies compounds for regrowing nerves, in live animals.
Publication Date:
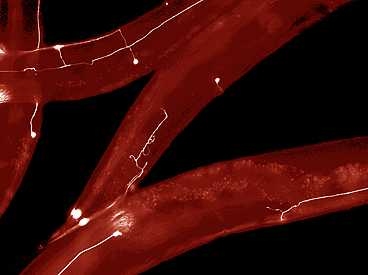
Caption:
MIT engineers have developed a way to rapidly perform surgery on single nerve cells in the worm C. elegans. The white lines represent axons — long extensions of nerve cells that carry messages to other cells.
Credits:
Image: Craig Millman and Yanik Lab





