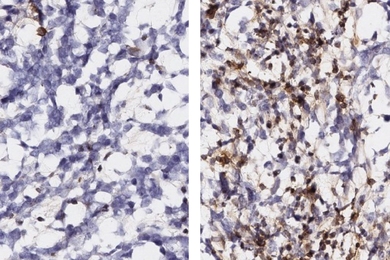
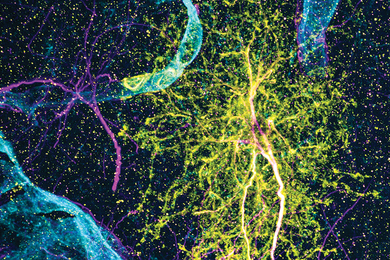
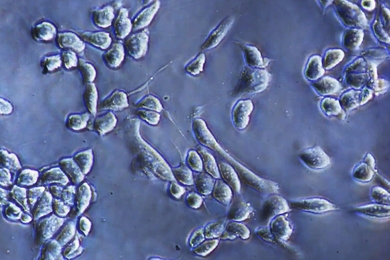
RNA editing study finds many ways for neurons to diversify
Tracking how fruit fly motor neurons edit their RNA, neurobiologists cataloged hundreds of target sites and varying editing rates, finding many edits altered communication- and function-related proteins.