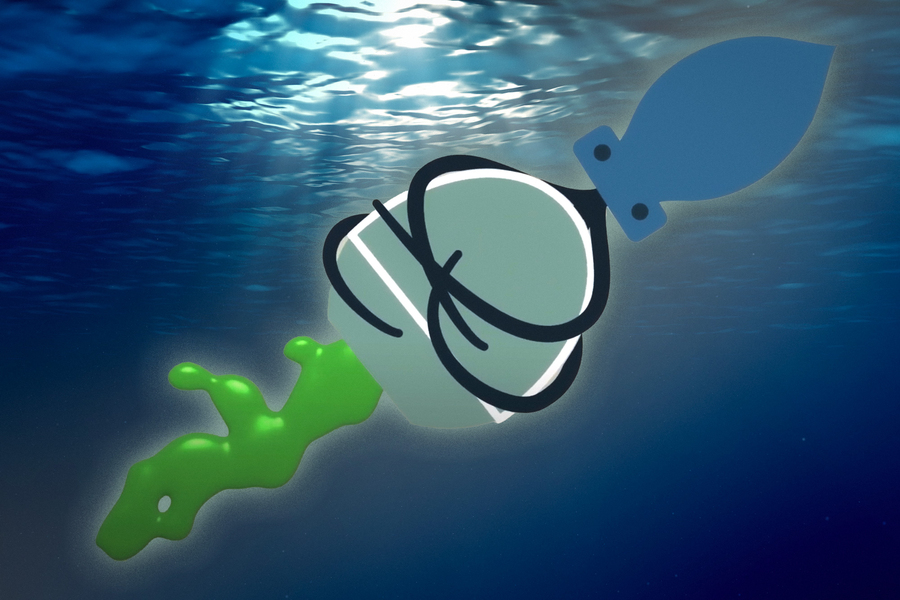
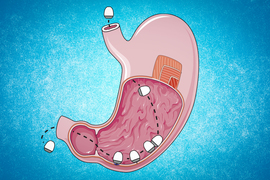
Inspired by the way that squids use jets to propel themselves through the ocean and shoot ink clouds, researchers from MIT and Novo Nordisk have developed an ingestible capsule that releases a burst of drugs directly into the wall of the stomach or other organs of the digestive tract.
This capsule could offer an alternative way to deliver drugs that normally have to be injected, such as insulin and other large proteins, including antibodies. This needle-free strategy could also be used to deliver RNA, either as a vaccine or a therapeutic molecule to treat diabetes, obesity, and other metabolic disorders.
“One of the longstanding challenges that we’ve been exploring is the development of systems that enable the oral delivery of macromolecules that usually require an injection to be administered. This work represents one of the next major advances in that progression,” says Giovanni Traverso, director of the Laboratory for Translational Engineering and an associate professor of mechanical engineering at MIT, a gastroenterologist at Brigham and Women’s Hospital, an associate member of the Broad Institute, and the senior author of the study.
Traverso and his students at MIT developed the new capsule along with researchers at Brigham and Women’s Hospital and Novo Nordisk. Graham Arrick SM ’20 and Novo Nordisk scientists Drago Sticker and Aghiad Ghazal are the lead authors of the paper, which appears today in Nature.
Inspired by cephalopods
Drugs that consist of large proteins or RNA typically can’t be taken orally because they are easily broken down in the digestive tract. For several years, Traverso’s lab has been working on ways to deliver such drugs orally by encapsulating them in small devices that protect the drugs from degradation and then inject them directly into the lining of the digestive tract.
Most of these capsules use a small needle or set of microneedles to deliver drugs once the device arrives in the digestive tract. In the new study, Traverso and his colleagues wanted to explore ways to deliver these molecules without any kind of needle, which could reduce the possibility of any damage to the tissue.
To achieve that, they took inspiration from cephalopods. Squids and octopuses can propel themselves by filling their mantle cavity with water, then rapidly expelling it through their siphon. By changing the force of water expulsion and pointing the siphon in different directions, the animals can control their speed and direction of travel. The siphon organ also allows cephalopods to shoot jets of ink, forming decoy clouds to distract predators.
The researchers came up with two ways to mimic this jetting action, using compressed carbon dioxide or tightly coiled springs to generate the force needed to propel liquid drugs out of the capsule. The gas or spring is kept in a compressed state by a carbohydrate trigger, which is designed to dissolve when exposed to humidity or an acidic environment such as the stomach. When the trigger dissolves, the gas or spring is allowed to expand, propelling a jet of drugs out of the capsule.
In a series of experiments using tissue from the digestive tract, the researchers calculated the pressures needed to expel the drugs with enough force that they would penetrate the submucosal tissue and accumulate there, creating a depot that would then release drugs into the tissue.
“Aside from the elimination of sharps, another potential advantage of high-velocity columnated jets is their robustness to localization issues. In contrast to a small needle, which needs to have intimate contact with the tissue, our experiments indicated that a jet may be able to deliver most of the dose from a distance or at a slight angle,” Arrick says.
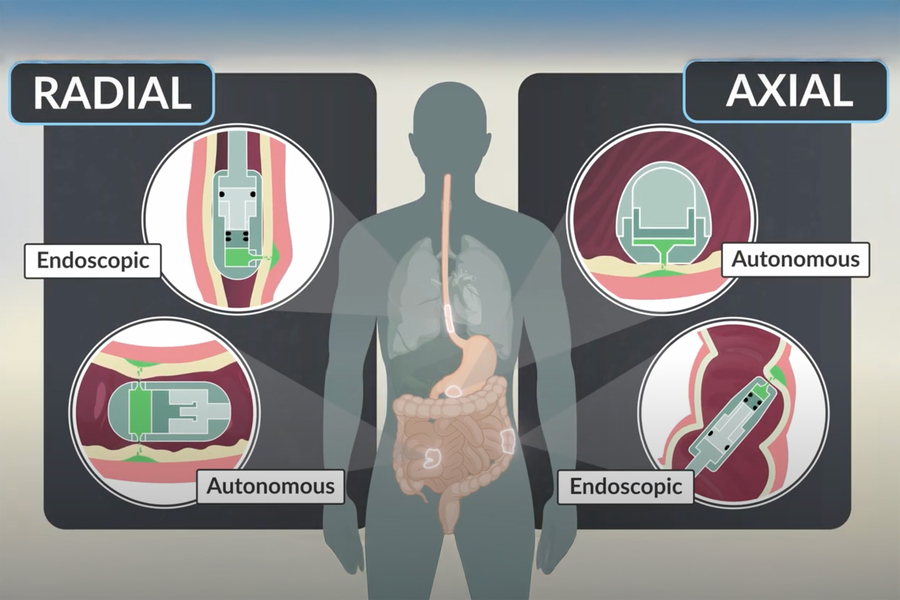
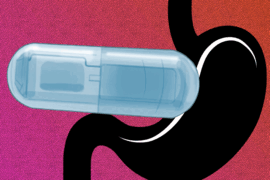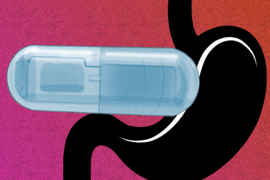
The researchers also designed the capsules so that they can target different parts of the digestive tract. One version of the capsule, which has a flat bottom and a high dome, can sit on a surface, such as the lining of the stomach, and eject drug downward into the tissue. This capsule, which was inspired by previous research from Traverso’s lab on self-orienting capsules, is about the size of a blueberry and can carry 80 microliters of drug.
The second version has a tube-like shape that allows it to align itself within a long tubular organ such as the esophagus or small intestine. In that case, the drug is ejected out toward the side wall, rather than downward. This version can deliver 200 microliters of drug.
Made of metal and plastic, the capsules can pass through the digestive tract and are excreted after releasing their drug payload.
Needle-free drug delivery
In tests in animals, the researchers showed that they could use these capsules to deliver insulin, a GLP-1 receptor agonist similar to the diabetes drug Ozempic, and a type of RNA called short interfering RNA (siRNA). This type of RNA can be used to silence genes, making it potentially useful in treating many genetic disorders.
They also showed that the concentration of the drugs in the animals’ bloodstream reached levels on the same order of magnitude as those seen when the drugs were injected with a syringe, and they did not detect any tissue damage.
The researchers envision that the ingestible capsule could be used at home by patients who need to take insulin or other injected drugs frequently. In addition to making it easier to administer drugs, especially for patients who don’t like needles, this approach also eliminates the need to dispose of sharp needles. The researchers also created and tested a version of the device that could be attached to an endoscope, allowing doctors to use it in an endoscopy suite or operating room to deliver drugs to a patient.
“This technology is a significant leap forward in oral drug delivery of macromolecule drugs like insulin and GLP-1 agonists. While many approaches for oral drug delivery have been attempted in the past, they tend to be poorly efficient in achieving high bioavailability. Here, the researchers demonstrate the ability to deliver bioavailability in animal models with high efficiency. This is an exciting approach which could be impactful for many biologics which are currently administered through injections or intravascular infusions,” says Omid Veiseh, a professor of bioengineering at Rice University, who was not involved in the research.
The researchers now plan to further develop the capsules, in hopes of testing them in humans.
The research was funded by Novo Nordisk, the Natural Sciences and Engineering Research Council of Canada, the MIT Department of Mechanical Engineering, Brigham and Women’s Hospital, and the U.S. Advanced Research Projects Agency for Health.