Every day, billions of red blood cells pass through the spleen, an organ that is responsible for filtering out old or damaged blood cells. This task is made more difficult when the blood cells are misshapen, as they are in patients with sickle cell disease, which affects millions of people throughout the world. Sickled blood cells can clog the spleen’s filters, leading to a potentially life-threatening situation.
Researchers at MIT, Nanyang Technological University in Singapore, the Pasteur Institute in Paris, and other institutions have now designed a microfluidic device, or “spleen-on-a-chip,” that can model how this phenomenon, known as acute splenic sequestration, arises.
The researchers found that low oxygen levels make it more likely that the spleen’s filters will become clogged. They also showed that boosting oxygen levels can unclog the filters, which may help to explain how blood transfusions help patients suffering from this condition.
“If we increase the oxygen levels, it will reverse the blockage,” says Ming Dao, a principal research scientist in MIT’s Department of Materials Science and Engineering and one of the senior authors of the study. “This mimics what’s done when there’s a splenic sequestration crisis. The first thing doctors do is transfusion, and in most cases, that gives some relief to the patient.”
Subra Suresh, former dean of engineering at MIT, the Vannevar Bush Professor Emeritus of Engineering, and former president of Singapore’s Nanyang Technological University; Pierre Buffet, medical director of the Pasteur Institute and a professor at the University of Paris; and George Karniadakis, the Robinson and Barstow Professor of Applied Mathematics at Brown University, are also senior authors of the study. MIT postdoc Yuhao Qiang is the lead author of the paper, which appears in Proceedings of the National Academy of Sciences this week.
Clogged filters
Most red blood cells have a lifespan of about 120 days, so nearly 1 percent of the supply has to be removed every day. Within the spleen, blood flows through tissue known as red pulp, which contains narrow passages called interendothelial slits.
These slits, formed by the spaces between the endothelial cells lining the spleen’s blood vessels, have maximum opening dimensions significantly smaller than those of a red blood cell. Any red blood cells that can’t pass through these tiny openings, because they’re damaged, stiffened or misshapen, become trapped and are destroyed by immune cells called macrophages.
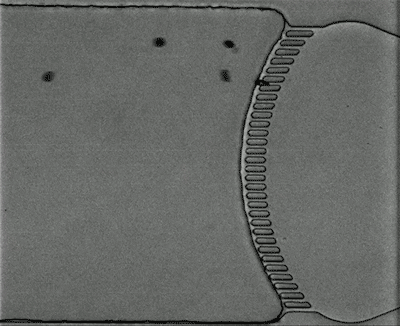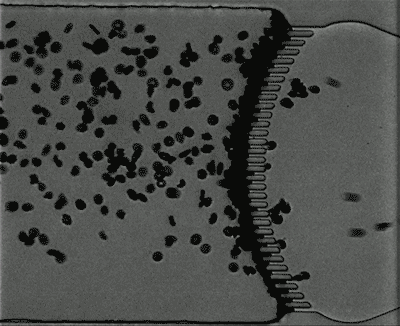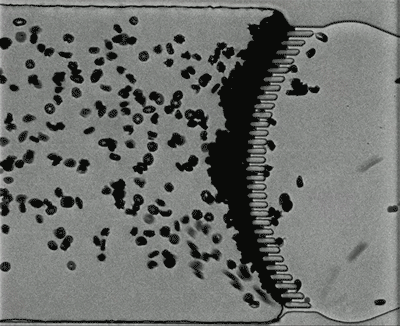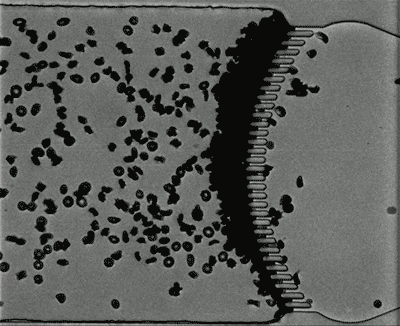
Credit: Courtesy of the researchers
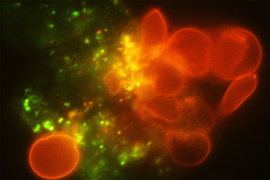
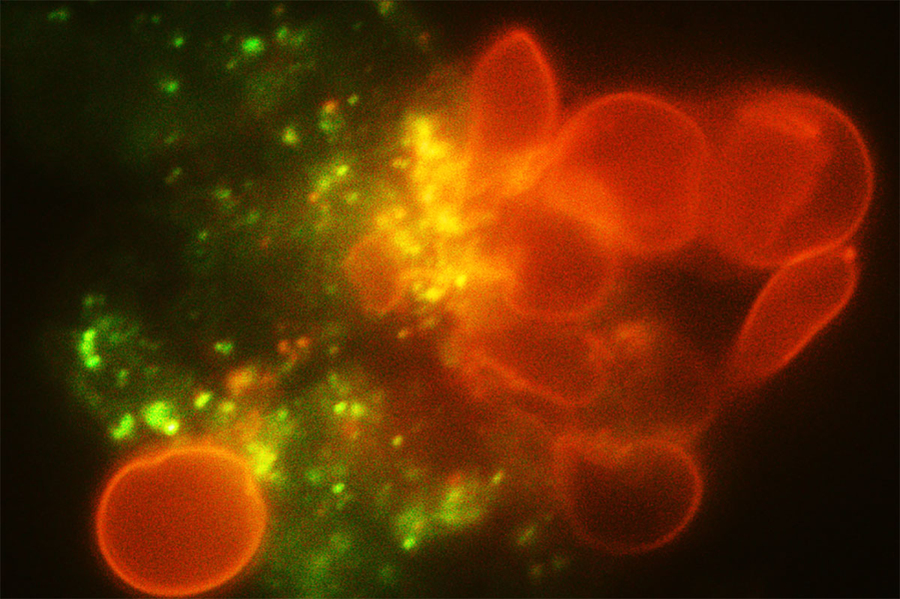
To model the spleen’s filtration function, the researchers created a microfluidic device with two modules — the S chip, which mimics the interendothelial slits, and the M chip, which mimics the macrophages. The device also includes a gas channel that can be used to control the oxygen concentration of each chip to simulate conditions in the body.
Using this device, the researchers sought to better understand acute splenic sequestration, which occurs in about 5 percent of patients with sickle cell disease, usually in children. When this happens, the spleen becomes enlarged, and the patient becomes severely anemic. Doctors usually treat it with blood transfusions, but if that doesn’t help, the spleen may need to be surgically removed.
Working with healthy red blood cells and sickled red cells from sickle cell disease patients, the researchers allowed the cells to flow through their device under controlled oxygen levels.
Under normal oxygen conditions (20 percent oxygen) sickled cells created some blockage at the slits, but there was still space for other blood cells to pass through. However, when the oxygen level decreased to 2 percent, the slits quickly became fully blocked.
When the researchers increased the oxygen level again, the blockage cleared up. This may partly explain why blood transfusions, which bring oxygenated blood cells into the spleen, can help patients who are experiencing acute splenic sequestration, Dao says.
“Our findings provide a general scientific framework to guide and rationalize what doctors observe. They also help to elucidate how the spleen provides a critical function to help filter blood cells,” Suresh says.
The researchers found that mildly deoxygenated conditions (5 percent oxygen) cause some clogging but not enough to produce a splenic sequestration crisis, which may explain why such crises occur rarely, Dao says.
Slow digestion
The researchers then used the other device module, the M chip, to model what happens as red blood cells encounter macrophages under different conditions. They found that when oxygen levels were low, sickled red blood cells were much more likely to be trapped by macrophages and ingested by them. In fact, so many blood cells were caught that macrophages became overwhelmed and couldn’t destroy them fast enough, contributing to the clogging of the slits.
The researchers also found that stiff sickled cells retained their sickled shape even after being ingested, which made it harder for macrophages to break them down. “About half of these cells stay sickled for a very long time and slow down the whole digestion process,” Dao says.
When oxygen levels were increased, the blood cells regained their normal shape, even the cells that had been ingested. This allowed macrophages to more easily digest them and clear up the clogged filters.
The researchers are now using the spleen-on-a-chip to study how drugs used to treat sickle cell disease, such as voxelotor and hydroxyurea, affect the cell behavior that they observed in this study. They also hope that the device could one day be used to help doctors analyze individual patients’ blood cells and monitor how their disease is progressing.
“This approach should help design assays to give patient-specific diagnosis and prognosis,” says Buffet, who is also a practicing clinician. “That may give doctors some idea of how well the patient is doing and in what situation they need to do a splenectomy or take other measures.”
The team also included MIT postdocs Ting Dong and Fuyin Zheng, Abdoulaye Sissoko from University of Paris, Zixiang Liu from Brown University, Fang Kong from Nanyang Technological University, and John Higgins from Massachusetts General Hospital. The research was funded by the National Institutes of Health and Nanyang Technological University.