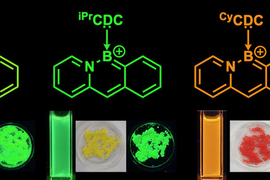
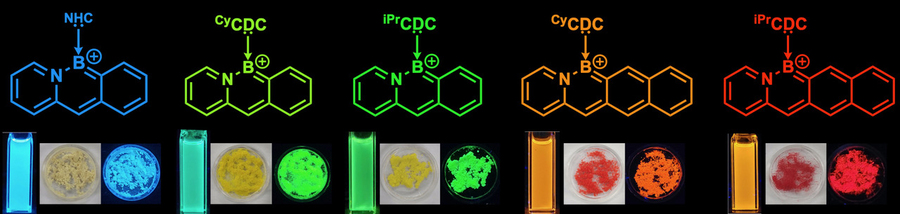
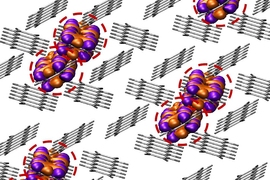
Chains of fused carbon-containing rings have unique optoelectronic properties that make them useful as semiconductors. These chains, known as acenes, can also be tuned to emit different colors of light, which makes them good candidates for use in organic light-emitting diodes.
The color of light emitted by an acene is determined by its length, but as the molecules become longer, they also become less stable, which has hindered their widespread use in light-emitting applications.
MIT chemists have now come up with a way to make these molecules more stable, allowing them to synthesize acenes of varying lengths. Using their new approach, they were able to build molecules that emit red, orange, yellow, green, or blue light, which could make acenes easier to deploy in a variety of applications.
“This class of molecules, despite their utility, have challenges in terms of their reactivity profile,” says Robert Gilliard, the Novartis Associate Professor of Chemistry at MIT and the senior author of the new study. “What we tried to address in this study first was the stability problem, and second, we wanted to make compounds where you could have a tunable range of light emission.”
MIT research scientist Chun-Lin Deng is the lead author of the paper, which appears today in Nature Chemistry.
Colorful molecules
Acenes consist of benzene molecules — rings made of carbon and hydrogen — fused together in a linear fashion. Because they are rich in sharable electrons and can efficiently transport an electric charge, they have been used as semiconductors and field-effect transistors (transistors that use an electric field to control the flow of current in a semiconductor).
Recent work has shown that acenes in which some of the carbon atoms are replaced, or “doped,” with boron and nitrogen have even more useful electronic properties. However, like traditional acenes, these molecules are unstable when exposed to air or light. Often, acenes have to be synthesized within a sealed container called a glovebox to protect them from air exposure, which can lead them to break down. The longer the acenes are, the more susceptible they are to unwanted reactions initiated by oxygen, water, or light.
To try to make acenes more stable, Gilliard decided to use a ligand that his lab has previously worked with, known as carbodicarbenes. In a study published last year, they used this ligand to stabilize borafluorenium ions, organic compounds that can emit different colors of light in response to temperature changes.
For this study, Gilliard and his co-authors developed a new synthesis that allowed them to add carbodicarbenes to acenes that are also doped with boron and nitrogen. With the addition of the new ligand, the acenes became positively charged, which improved their stability and also gave them unique electronic properties.
Using this approach, the researchers created acenes that produce different colors, depending on their length and the types of chemical groups attached to the carbodicarbene. Until now, most of the boron, nitrogen-doped acenes that had been synthesized could emit only blue light.
“Red emission is very important for wide-ranging applications, including biological applications like imaging,” Gilliard says. “A lot of human tissue emits blue light, so it’s difficult to use blue-fluorescent probes for imaging, which is one of the many reasons why people are looking for red emitters.”
Better stability
Another important feature of these acenes is that they remain stable in both air and water. Boron-containing charged molecules with a low coordination number (meaning the central boron atom has few neighbors) are often highly unstable in water, so the acenes’ stability in water is notable and could make it feasible to use them for imaging and other medical applications.
“One of the reasons why we're excited about the class of compounds that we're reporting in this paper is that they can be suspended in water. That opens up a wide range of possibilities,” Gilliard says.
The researchers now plan to try incorporating different types of carbodicarbenes to see if they can create additional acenes with even better stability and quantum efficiency (a measure of how much light is emitted from the material).
“We think it will be possible to make a lot of different derivatives that we haven't even synthesized yet,” Gilliard says. “There are a lot of optoelectronic properties that can be dialed in that we have yet to explore, and we're excited about that as well.”
Gilliard also plans to work with Marc Baldo, an MIT professor of electrical engineering, to try incorporating the new acenes into a type of solar cell known as a single-fission-based solar cell. This type of solar cell can produce two electrons from one photon, making the cell much more efficient.
These types of compounds could also be developed for use as light-emitting diodes for television and computer screens, Gilliard says. Organic light-emitting diodes are lighter and more flexible than traditional LEDs, produce brighter images, and consume less power.
“We're still in the very early stages of developing the specific applications, whether it's organic semiconductors, light-emitting devices, or singlet-fission-based solar cells, but due to their stability, the device fabrication should be much smoother than typical for these kinds of compounds,” Gilliard says.
“By combining reactive zerovalent carbon and cationic boron species, this creative work with a nontraditional paradigm certainly paves a promising path toward the development of highly air- and photo-stable light-emitting materials and miniature energy harvesting devices,” says Tiow-Gan Ong, deputy director of the Institute of Chemistry at the Academia Sinica, who was not involved in the research.
The research was funded by the Arnold and Mabel Beckman Foundation and the National Science Foundation Major Research Instrumentation Program.