Singapore-MIT Alliance for Research and Technology (SMART), MIT’s research enterprise in Singapore, has received a grant from the National Medical Research Council (NMRC) Covid-19 Research fund for the acceleration of research and development of rapid paper-based serological and diagnostic tests for Covid-19. The one-year grant is part of NMRC’s support for Covid-19 related research, and will focus on developing rapid tests based on protein detection that will overcome challenges and bottlenecks faced by existing diagnostic tests.
As governments worldwide continue to wage war against the novel coronavirus, and with the widespread implementation of treatment and vaccines more than a year away, an important approach in the multi-pronged strategy to fight the pandemic is the ability to rapidly detect and treat infected individuals to prevent exponential spreading. Presymptomatic and asymptomatic transmissions present difficult challenges in containing the virus.
Even as dozens of diagnostic tests, based on RNA or antibody detection, for Covid-19 have been made available, major challenges remain. Current diagnostic tests require health workers wearing substantial personal protective equipment to collect swabs. Afterwards, skilled personnel and speciality laboratory facilities are needed to prepare samples, run assays, and detect assay signals. This restricts diagnostic capabilities to hospitals and specialized diagnostic laboratories. In addition, assay times for existing genomic profile and serological tests can take from three hours to as long as five days. False negatives are also often observed in genomic profile tests due to low viral loads from throat swab samples.
“Testing of the SARS-CoV-2 virus is important to complement efforts in the fight against the Covid-19 pandemic,” says Professor Peter Preiser, associate vice president for biomedical and life sciences at Nanyang Technological University (NTU), and co-lead principal investigator at SMART's Antimicrobial Resistance (AMR) Interdisciplinary Research Group (IRG). “We are developing protein tests that may overcome existing challenges faced in Singapore and countries worldwide. These tests could be used by anyone, anywhere, with an almost immediate turnaround time for results. Think rapid influenza test kits, but for Covid-19, the paper-based tests will change from white to blue when molecules of the SARS-CoV-2 virus are detected from bodily fluids such as the saliva.”
Researchers from SMART AMR, NTU, and MIT are working collaboratively to develop a rapid serological test and a rapid diagnostic test (RDT), based on protein detection, that could be used outside of laboratories, without specialised equipment or infrastructure, at places such as airports and community clinics. Tests can be easily administered by anyone and will be stable in field conditions with results expected within 10 minutes. When SARS-CoV-2 virus is detected on the paper-based tests, the strips will change from white to blue to indicate positive for Covid-19.
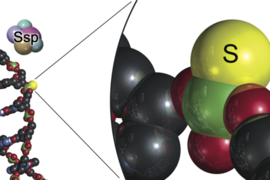
The paper-based serological test, using S protein, will detect SARS-CoV-2 antibodies from blood or serum samples. S protein, which is much smaller than an antibody, allows for high-density capture of antigens. Cellulose offers fast flow rates and low non-specific binding, yielding greater sensitivity and faster testing at a lower cost than standard polystyrene 96 well plate serological tests. With the ability to detect past and active infections, the test will help determine the realistic number of SARS-CoV-2 infected cases to understand the true magnitude of the pandemic.
The paper-based RDT, using S and N protein, will detect SARS-CoV-2 biomarkers from swabs or bodily fluids such as saliva or blood. The ability to test for N protein, which is observed from as early as Day 1 of disease onset, means that the RDT may be able to perform first-line screening for the virus, even for mild infections — an important feature that can help speed up containment efforts.
“Years of investment into the research of diagnostic surveillance and development of tests to combat the threat of antimicrobial resistance have allowed us to adapt the research and technologies for use in our diagnostic tests for Covid-19,” shares Professor Hadley Sikes, principal investigator at SMART AMR, and associate professor at MIT. “Based on past research on the SARS-CoV virus, we have chosen the Spike (S) protein and Nucleocapsid (N) protein as targets, with the latter observed in serum samples from as early as Day 1 of disease onset. Using our RDT, we will further determine whether presymptomatic and asymptomatic cases of infections can be detected.”
The new rapid tests build upon existing research on diagnostic assays from SMART AMR and the Sikes lab at MIT. The existing protein engineering technology is used for detection of other well-known biomarkers such as the Zika virus (ZNS1), tuberculosis (RV1656), and proinflammatory cytokine (IL-6). Using reduced-charge Sso7d, a 7-kDa, heat-stable, repurposed DNA-binding protein modified from Sulfolobus solfataricus, the developed diagnostic tests for SARS-CoV-2 will have superior stability properties even in conditions of high humidity, up to 98 degrees Celsius, and across a wide pH range.
The next phase of the research will focus on the optimization of fusion proteins that enable high-performance paper-based immunoassays, the directed evolution of binding proteins, and the evaluation of both serology and viral protein tests using clinical samples. Broader usage and implementation of both tests are also being explored.
“Collaborations with NTU, MIT, and amongst the five IRGs within SMART have allowed us to tap on the wide range of disciplines and expertise required to develop the tests in a short amount of time,” says Sikes. “With full focus on the research and development of the tests and support by our partners in Singapore, we’re grateful for the NMRC grant that will help us tremendously. We’ve begun discussions with interested companies on licensing and manufacturing of the serological test and RDT to enable broader testing and use.”
The research team is led by Preiser, principal investigator; Patthara Kongsuphol, co-investigator; and collaborator Sikes. It is funded by National Research Foundation Singapore under its Campus for Research Excellence and Technological Enterprise program, and NMRC under its Covid-19 Research Fund.
The AMR IRG is a translational research and entrepreneurship program that tackles the growing threat of antimicrobial resistance. By leveraging talent and convergent technologies across Singapore and MIT, they aim to tackle AMR head-on by developing multiple innovative and disruptive approaches to identify, respond to, and treat drug-resistant microbial infections. Through strong scientific and clinical collaborations, their goal is to provide transformative, holistic solutions for Singapore and the world.
SMART was established by MIT in partnership with the National Research Foundation Singapore in 2007. SMART is the first entity in the Campus for Research Excellence and Technological Enterprise (CREATE) developed by the National Research Foundation Singapore. SMART serves as an intellectual and innovation hub for research interactions between MIT and Singapore. Cutting-edge research projects in areas of interest to both Singapore and MIT are undertaken at SMART. SMART currently comprises an Innovation Center and five Interdisciplinary Research Groups (IRGs): Antimicrobial Resistance, Critical Analytics for Manufacturing Personalized-Medicine, Disruptive and Sustainable Technologies for Agricultural Precision, Future Urban Mobility, and Low Energy Electronic Systems. SMART research is funded by the National Research Foundation Singapore under the CREATE program.