In recent decades, the search for high-performance thermal insulation for buildings has prompted manufacturers to turn to aerogels. Invented in the 1930s, these remarkable materials are translucent, ultraporous, lighter than a marshmallow, strong enough to support a brick, and an unparalleled barrier to heat flow, making them ideal for keeping heat inside on a cold winter day and outside when summer temperatures soar.
Five years ago, researchers led by Evelyn Wang, a professor and head of the Department of Mechanical Engineering, and Gang Chen, the Carl Richard Soderberg Professor in Power Engineering, set out to add one more property to that list. They aimed to make a silica aerogel that was truly transparent.
“We started out trying to realize an optically transparent, thermally insulating aerogel for solar thermal systems,” says Wang. Incorporated into a solar thermal collector, a slab of aerogel would allow sunshine to come in unimpeded but prevent heat from coming back out — a key problem in today’s systems. And if the transparent aerogel were sufficiently clear, it could be incorporated into windows, where it would act as a good heat barrier but still allow occupants to see out.
When the researchers started their work, even the best aerogels weren’t up to those tasks. “People had known for decades that aerogels are a good thermal insulator, but they hadn’t been able to make them very optically transparent,” says Lin Zhao PhD ’19 of mechanical engineering. “So in our work, we’ve been trying to understand exactly why they’re not very transparent, and then how we can improve their transparency.”
Aerogels: opportunities and challenges
The remarkable properties of a silica aerogel are the result of its nanoscale structure. To visualize that structure, think of holding a pile of small, clear particles in your hand. Imagine that the particles touch one another and slightly stick together, leaving gaps between them that are filled with air. Similarly, in a silica aerogel, clear, loosely connected, nanoscale silica particles form a three-dimensional solid network within an overall structure that is mostly air. Because of all that air, a silica aerogel has an extremely low density — in fact, one of the lowest densities of any known bulk material — yet it’s solid and structurally strong, though brittle.
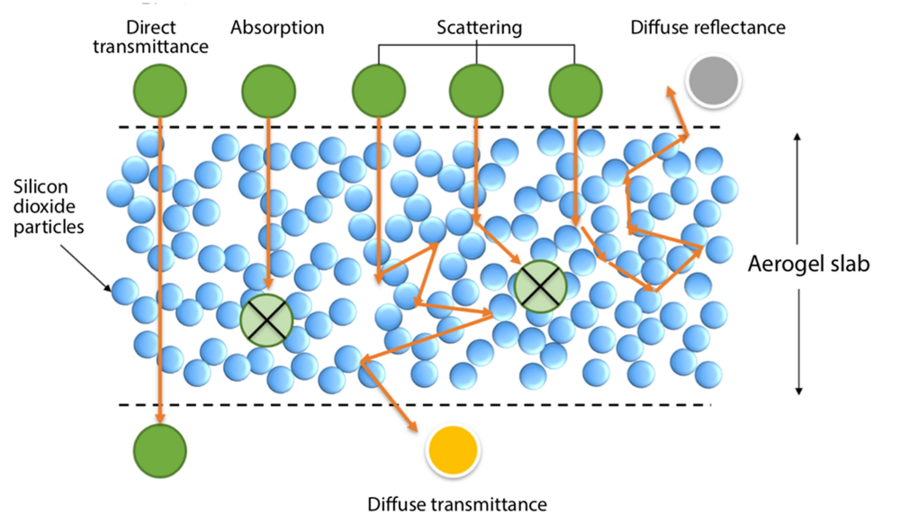
If a silica aerogel is made of transparent particles and air, why isn’t it transparent? Because the light that enters doesn’t all pass straight through. It is diverted whenever it encounters an interface between a solid particle and the air surrounding it. Figure 1 in the slideshow above illustrates the process. When light enters the aerogel, some is absorbed inside it. Some — called direct transmittance — travels straight through. And some is redirected along the way by those interfaces. It can be scattered many times and in any direction, ultimately exiting the aerogel at an angle. If it exits from the surface through which it entered, it is called diffuse reflectance; if it exits from the other side, it is called diffuse transmittance.
To make an aerogel for a solar thermal system, the researchers needed to maximize the total transmittance: the direct plus the diffuse components. And to make an aerogel for a window, they needed to maximize the total transmittance and simultaneously minimize the fraction of the total that is diffuse light. “Minimizing the diffuse light is critical because it’ll make the window look cloudy,” says Zhao. “Our eyes are very sensitive to any imperfection in a transparent material.”
Developing a model
The sizes of the nanoparticles and the pores between them have a direct impact on the fate of light passing through an aerogel. But figuring out that interaction by trial and error would require synthesizing and characterizing too many samples to be practical. “People haven’t been able to systematically understand the relationship between the structure and the performance,” says Zhao. “So we needed to develop a model that would connect the two.”
To begin, Zhao turned to the radiative transport equation, which describes mathematically how the propagation of light (radiation) through a medium is affected by absorption and scattering. It is generally used for calculating the transfer of light through the atmospheres of Earth and other planets. As far as Wang knows, it has not been fully explored for the aerogel problem.
Both scattering and absorption can reduce the amount of light transmitted through an aerogel, and light can be scattered multiple times. To account for those effects, the model decouples the two phenomena and quantifies them separately — and for each wavelength of light.
Based on the sizes of the silica particles and the density of the sample (an indicator of total pore volume), the model calculates light intensity within an aerogel layer by determining its absorption and scattering behavior using predictions from electromagnetic theory. Using those results, it calculates how much of the incoming light passes directly through the sample and how much of it is scattered along the way and comes out diffuse.
The next task was to validate the model by comparing its theoretical predictions with experimental results.
Synthesizing aerogels
Working in parallel, graduate student Elise Strobach of mechanical engineering had been learning how best to synthesize aerogel samples — both to guide development of the model and ultimately to validate it. In the process, she produced new insights on how to synthesize an aerogel with a specific desired structure.
Her procedure starts with a common form of silicon called silane, which chemically reacts with water to form an aerogel. During that reaction, tiny nucleation sites occur where particles begin to form. How fast they build up determines the end structure. To control the reaction, she adds a catalyst, ammonia. By carefully selecting the ammonia-to-silane ratio, she gets the silica particles to grow quickly at first and then abruptly stop growing when the precursor materials are gone — a means of producing particles that are small and uniform. She also adds a solvent, methanol, to dilute the mixture and control the density of the nucleation sites, thus the pores between the particles.
The reaction between the silane and water forms a gel containing a solid nanostructure with interior pores filled with the solvent. To dry the wet gel, Strobach needs to get the solvent out of the pores and replace it with air — without crushing the delicate structure. She puts the aerogel into the pressure chamber of a critical point dryer and floods liquid CO2 into the chamber. The liquid CO2 flushes out the solvent and takes its place inside the pores. She then slowly raises the temperature and pressure inside the chamber until the liquid CO2 transforms to its supercritical state, where the liquid and gas phases can no longer be differentiated. Slowly venting the chamber releases the CO2 and leaves the aerogel behind, now filled with air. She then subjects the sample to 24 hours of annealing — a standard heat-treatment process — which slightly reduces scatter without sacrificing the strong thermal insulating behavior. Even with the 24 hours of annealing, her novel procedure shortens the required aerogel synthesis time from several weeks to less than four days.
Validating and using the model
To validate the model, Strobach fabricated samples with carefully controlled thicknesses, densities, and pore and particle sizes — as determined by small-angle X-ray scattering — and used a standard spectrophotometer to measure the total and diffuse transmittance.
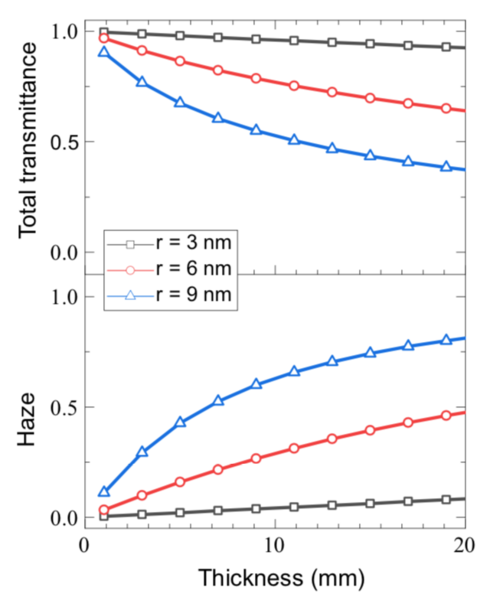
The data confirmed that, based on measured physical properties of an aerogel sample, the model could calculate total transmittance of light as well as a measure of clarity called haze, defined as the fraction of total transmittance that is made up of diffuse light.
The exercise confirmed simplifying assumptions made by Zhao in developing the model. Also, it showed that the radiative properties are independent of sample geometry, so his model can simulate light transport in aerogels of any shape. And it can be applied not just to aerogels, but to any porous materials.
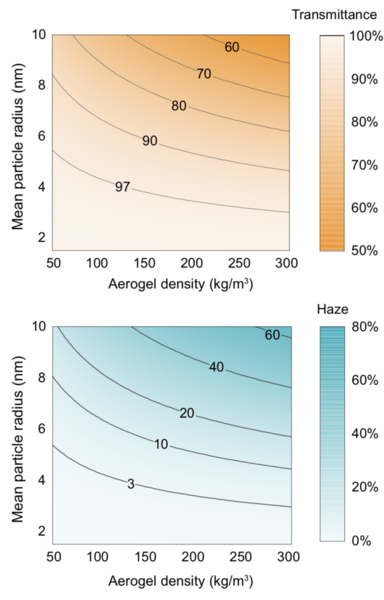
Wang notes what she considers the most important insight from the modeling and experimental results: “Overall, we determined that the key to getting high transparency and minimal haze — without reducing thermal insulating capability — is to have particles and pores that are really small and uniform in size,” she says.
One analysis demonstrates the change in behavior that can come with a small change in particle size. Many applications call for using a thicker piece of transparent aerogel to better block heat transfer. But increasing thickness may decrease transparency. With their samples, as long as particle size is small, increasing thickness to achieve greater thermal insulation will not significantly decrease total transmittance or increase haze.
Comparing aerogels from MIT and elsewhere
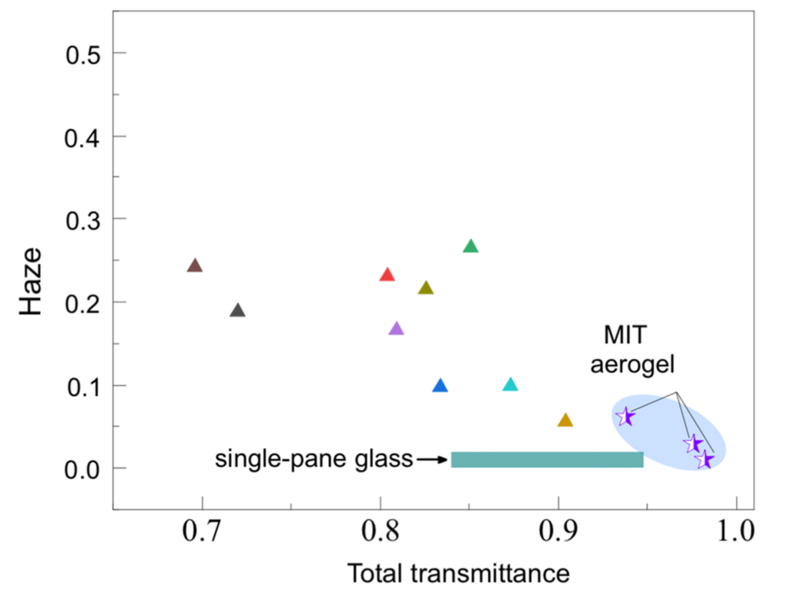
How much difference does their approach make? “Our aerogels are more transparent than glass because they don’t reflect — they don’t have that glare spot where the glass catches the light and reflects to you,” says Strobach.
To Lin, a main contribution of their work is the development of general guidelines for material design, as demonstrated by Figure 4 in the slideshow above. Aided by such a “design map,” users can tailor an aerogel for a particular application. Based on the contour plots, they can determine the combinations of controllable aerogel properties — namely, density and particle size — needed to achieve a targeted haze and transmittance outcome for many applications.
Aerogels in solar thermal collectors
The researchers have already demonstrated the value of their new aerogels for solar thermal energy conversion systems, which convert sunlight into thermal energy by absorbing radiation and transforming it into heat. Current solar thermal systems can produce thermal energy at so-called intermediate temperatures — between 120 and 220 degrees Celsius — which can be used for water and space heating, steam generation, industrial processes, and more. Indeed, in 2016, U.S. consumption of thermal energy exceeded the total electricity generation from all renewable sources.
However, state-of-the-art solar thermal systems rely on expensive optical systems to concentrate the incoming sunlight, specially designed surfaces to absorb radiation and retain heat, and costly and difficult-to-maintain vacuum enclosures to keep that heat from escaping. To date, the costs of those components have limited market adoption.
Zhao and his colleagues thought that using a transparent aerogel layer might solve those problems. Placed above the absorber, it could let through incident solar radiation and then prevent the heat from escaping. So it would essentially replicate the natural greenhouse effect that’s causing global warming — but to an extreme degree, on a small scale, and with a positive outcome.
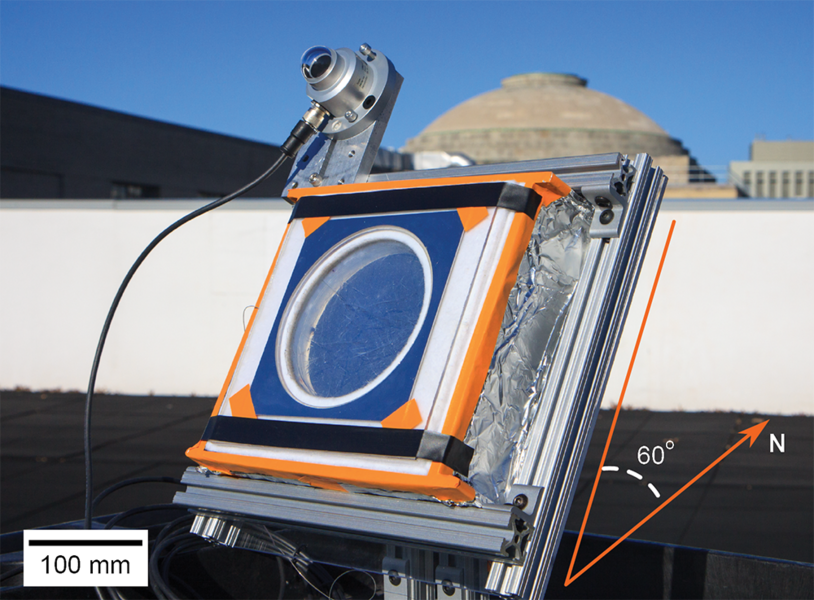
To try it out, the researchers designed an aerogel-based solar thermal receiver. The device consists of a nearly “blackbody” absorber (a thin copper sheet coated with black paint that absorbs all radiant energy that falls on it), and above it a stack of optimized, low-scattering silica aerogel blocks, which efficiently transmit sunlight and suppress conduction, convection, and radiation heat losses simultaneously. The nanostructure of the aerogel is tailored to maximize its optical transparency while maintaining its ultralow thermal conductivity. With the aerogel present, there is no need for expensive optics, surfaces, or vacuum enclosures.
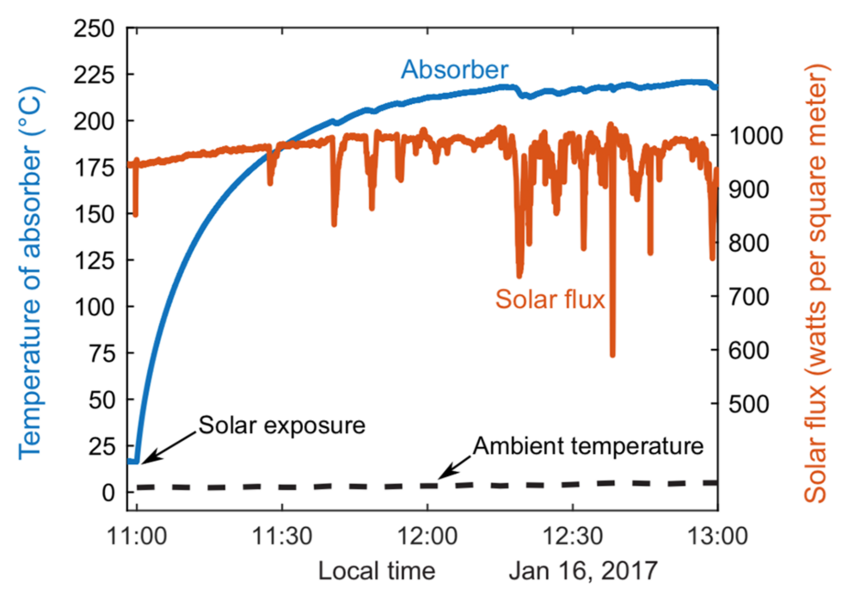
After extensive laboratory tests of the device, the researchers decided to test it “in the field” — in this case, on the roof of an MIT building. On a sunny day in winter, they set up their device, fixing the receiver toward the south and tilted 60 degrees from horizontal to maximize solar exposure. They then monitored its performance between 11 a.m. and 1 p.m. Despite the cold ambient temperature (less than 1 C) and the presence of clouds in the afternoon, the temperature of the absorber started increasing right away and eventually stabilized above 220 C.
To Zhao, the performance already demonstrated by the artificial greenhouse effect opens up what he calls “an exciting pathway to the promotion of solar thermal energy utilization.” Already, he and his colleagues have demonstrated that it can convert water to steam that is greater than 120 C. In collaboration with researchers at the Indian Institute of Technology Bombay, they are now exploring possible process steam applications in India and performing field tests of a low-cost, completely passive solar autoclave for sterilizing medical equipment in rural communities.
Windows and more
Strobach has been pursuing another promising application for the transparent aerogel — in windows. “In trying to make more transparent aerogels, we hit a regime in our fabrication process where we could make things smaller, but it didn’t result in a significant change in the transparency,” she says. “But it did make a significant change in the clarity,” a key feature for a window.
The availability of an affordable, thermally insulating window would have several impacts, says Strobach. Every winter, windows in the United States lose enough energy to power over 50 million homes. That wasted energy costs the economy more than $32 billion a year and generates about 350 million tons of CO2 — more than is emitted by 76 million cars. Consumers can choose high-efficiency triple-pane windows, but they’re so expensive that they’re not widely used.
Analyses by Strobach and her colleagues showed that replacing the air gap in a conventional double-pane window with an aerogel pane could be the answer. The result could be a double-pane window that is 40 percent more insulating than traditional ones and 85 percent as insulating as today’s triple-pane windows — at less than half the price. Better still, the technology could be adopted quickly. The aerogel pane is designed to fit within the current two-pane manufacturing process that’s ubiquitous across the industry, so it could be manufactured at low cost on existing production lines with only minor changes.
Guided by Zhao’s model, the researchers are continuing to improve the performance of their aerogels, with a special focus on increasing clarity while maintaining transparency and thermal insulation. In addition, they are considering other traditional low-cost systems that would — like the solar thermal and window technologies — benefit from sliding in an optimized aerogel to create a high-performance heat barrier that lets in abundant sunlight.
This research was supported by the Full-Spectrum Optimized Conversion and Utilization of Sunlight program of the U.S. Department of Energy’s Advanced Research Projects Agency–Energy; the Solid-State Solar Thermal Energy Conversion Center, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences; and the MIT Tata Center for Technology and Design. Elise Strobach received funding from the National Science Foundation Graduate Research Fellowship Program. Lin Zhao PhD ’19 is now an optics design engineer at 3M in St. Paul, Minnesota.
This article appears in the Autumn 2019 issue of Energy Futures, the magazine of the MIT Energy Initiative.