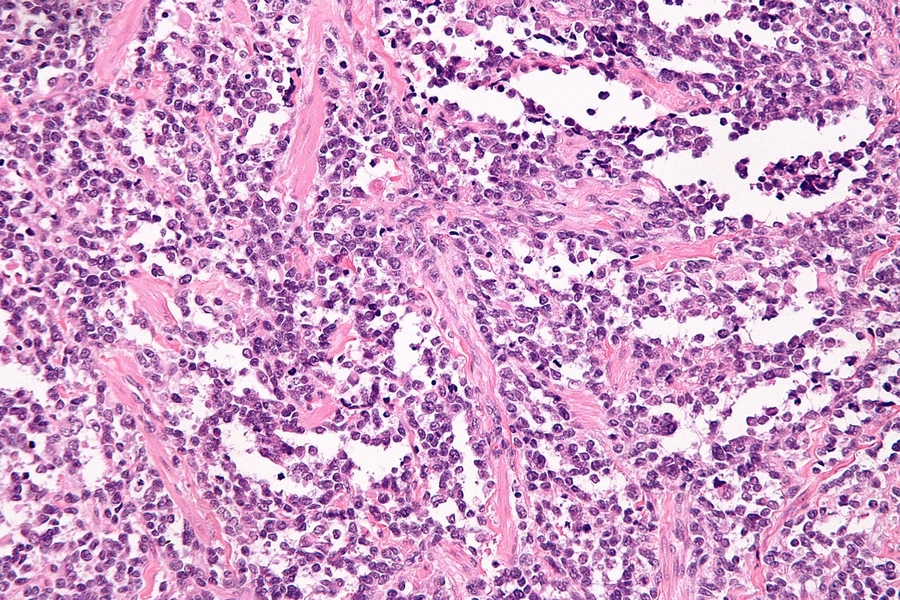
MIT Professor Angela Koehler is part of a team that has been awarded a federal grant to study one of the least-understood but most-fatal forms of childhood cancer, fusion-positive alveolar rhabdomyosarcoma (ARMS).
The $5.8 million, five-year grant is part of the Cancer Moonshot Initiative, a National Institutes of Health program dedicating $1.8 billion over seven years to accelerating the discovery of new ways to prevent, diagnose, and cure cancer. Koehler, the Samuel A. Goldblith Career Development Professor in Applied Biology and a member of MIT’s Koch Institute for Integrative Cancer Research, will be part of an international team led by researchers from the Broad Institute of MIT and Harvard, Duke University, the National Cancer Institute, and the University of Zurich.
ARMS, a cancer affecting skeletal muscle tissue, is rare, accounting for about 1 percent of all cancers among children and adolescents, and an annual incidence that is truly “one in a million.” It is also deadly: The overall survival rate for fusion-positive ARMS is 30 percent, with only a 10 percent survival rate for patients whose cancer has metastasized. Currently, there are no ARMS-specific therapies available. As a poorly understood “orphan disease,” it would be difficult for the pharmaceutical industry to undertake the costly development of new treatments for such a small pool of patients.
The new grant will enable Koehler and her team to make significant strides toward improving our understanding of the molecular underpinnings of fusion-positive ARMS and identifying compounds that could be developed into new treatments.
“Fusion oncoproteins involving transcription factors are the holy grail for drug discovery, and nearly all traditional drug discovery approaches have failed,” says Koehler. “By combining new technology approaches, we may have cracked the door open on one of these targets. This high-risk, high-reward grant will enable us to develop a current lead and develop new approaches to the wider set of oncoproteins studied by the NCI Network.”
Fusion oncoproteins — proteins that result from a complex mutation joining two genes together — drive many childhood cancers. In fusion-positive ARMS, the most common culprit is a chimera of transcription factors PAX3 and FOXO1, two proteins that are part of the molecular machinery that regulates the expression of genes.
Unlike many other oncoproteins, which may be present in both cancerous and normal cells, fusion oncoproteins like PAX3-FOXO1 are present only in cancer cells. Drugs that target PAX3-FOXO1 have the potential to attack the root cause of cancer development while leaving healthy cells undamaged.
Yet PAX3-FOXO1 is considered an “undruggable” target. Like other transcription factors, its disordered nature resists conventional methods for studying structure, such as crystallography. Without comprehensive knowledge of the structure, it is challenging to design a new compound that will interfere with its function. Furthermore, transcription factors tend to lack the small, well-defined binding pockets that serve as the “lock” for the small-molecule “key” identified in high-throughput screens that use traditional binding assays.
Koehler will co-lead a project with Beat Schaefer of the University of Zurich to screen for new agents that block PAX3-FOXO1 activity. Her lab specializes in small-molecule microarray (SMM) platforms capable of identifying small molecules with multiple modes of binding, regardless of how disordered or intractable a target may be. Koehler has had promising results from pilot SMM screens of PAX3-FOXO1 and success with the same approach applied to other supposedly undruggable targets. The team will assess how binding compounds of interest change PAX3-FOXO1 activity, and optimize them to be more effective. The most promising candidate will then be tested in cell lines and mouse models.
If the project succeeds in targeting PAX3-FOXO1, the resulting probes — which will be made freely available to the wider research community — could serve as a starting point for developing new therapies for fusion-positive ARMS, as well as a tool for studying how PAX3-FOXO1 interacts with other proteins and DNA. But applications of the project’s results could extend well beyond this one orphan disease. The approaches developed could be used to identify therapeutic targets for other fusion-positive cancers, such as Ewing’s sarcoma and acute myeloid leukemia, and inform strategies for targeting oncogenic transcription factors more broadly.
“Given the relatively small patient populations, it may be challenging for our colleagues in the pharmaceutical industry to undertake drug discovery campaigns for these targets,” says Koehler. “We can and should take that kind of risk in my lab, given the great unmet need for these pediatric patients. Hopefully, our work will uncover leads that can be developed for translation, as well as lower the barrier for pharmaceutical companies to go after these as-of-yet undrugged targets. The trainees in our lab are very excited about this challenge and hope our work can impact the lives of children with ARMS.”