MIT associate professor of metallurgy Antoine Allanore has received a $1.9 million grant from the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE) to run larger scale tests of a new way to produce copper using electricity to separate copper from melted sulfur-based minerals, which are the main source of copper.
One of Allanore's primary goals is to make high-purity copper that can go directly into production of copper wire, which is in increasing demand for applications from renewable energy to electric vehicles. Production of electric and hybrid cars and buses is expected to rise from 3.1 million vehicles in 2017 to 27.2 million by 2027, with an accompanying nine-fold increase in demand for copper from 204,000 metric tons to 1.9 million metric tons (2.09 million U.S. tons) over the same period, according to a March 2017 IDTechEx report commissioned by the International Copper Association (ICA).
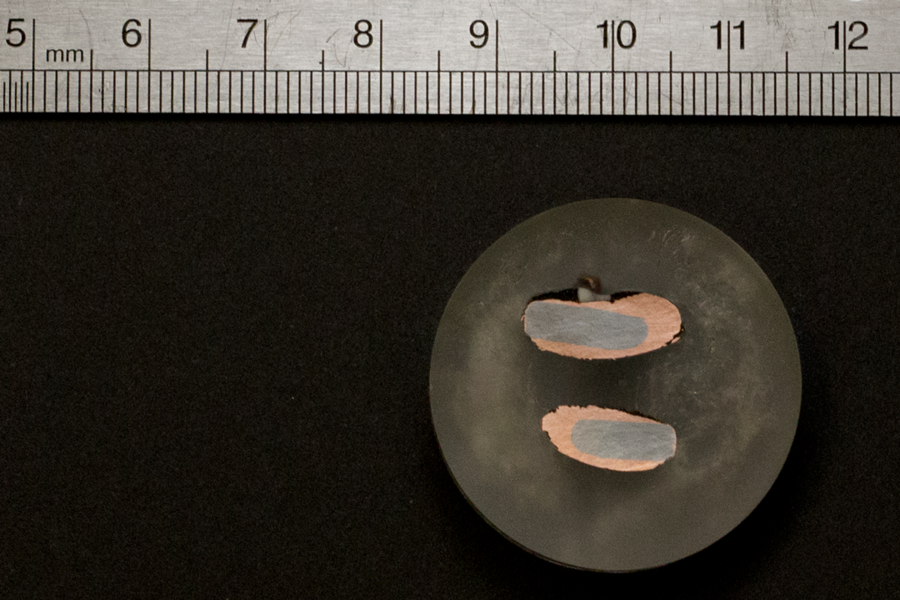
In June 2017, researchers in Allanore’s lab identified how to selectively separate pure copper and other metallic elements from sulfide mineral ore in one step. Their molten sulfide electrolysis process eliminates sulfur dioxide, a noxious byproduct of traditional copper extraction methods, instead producing pure elemental sulfur.
“We think that with our technology we could provide these copper wires with less energy consumption and higher productivity,” Allanore says. It may be possible to cut the energy needed for making copper by 20 percent.
In earlier research, postdoc Sulata K. Sahu and graduate student Brian J. Chmielowiec ’12, decomposed sulfur-rich minerals at high temperature into pure sulfur and extracted three different metals at very high purity: copper, molybdenum, and rhenium. The process is similar to the Hall-Héroult process, which uses electrolysis to produce aluminum, but operates at a higher operating temperature to enable production of liquid copper.
Currently, it takes multiple steps to separate out copper, first crushing sulfide minerals, and then floating out the copper-bearing parts. This copper-rich material — copper concentrate — is next partially refined in a smelter, and further purified with electrolytic refining. “Professor Allanore’s approach would work on the copper concentrate and has the potential to produce copper rod in a single operation while separating unwanted impurities and recovering valuable byproducts that are also in the concentrate,” says Hal Stillman, director of technology development and transfer for the International Copper Association. “Professor Allanore’s approach is a big step; it allows a completely new approach to refining copper.”
The three-year, $1.89 million DOE award will allow Allanore’s group to make a larger reactor, producing about 10 times as much liquid copper per hour, and to run the reactor for a longer time, enough to identify what happens to the other metals accompanying copper, which are also commercially important.
Allanore’s group effort began this year, and he hopes it will provide the data needed to move on to a pilot plant within three years. “We are aiming to be ready to provide the design criteria, the material and operating conditions of a one metric ton per day demonstration reactor,” Allanore says. “If everything is successful, that’s what we will deliver.”
Key technical challenges to overcome are proving the durability of the process over a longer time period and verifying the purity of the metals that are made in the process. Some of the byproducts of copper production, selenium, for example, are valuable in their own right.
“The revolution that we are proposing is that only one reactor would do everything. It would make the liquid copper product and allow us to recover elemental sulfur, and allows us to recover selenium,” Allanore says. “We are using electricity, and electrons can be very selective, so we are using electrons in a manner that enables the most efficient separation of the products of the chemical process.”
Conventional pyrometallurgy produces copper by burning the ore in air, requires four steps and produces noxious compounds like sulfur dioxide (SO2) that require secondary processing into sulfuric acid. The initial batch of copper also requires further processing. “It leaves behind copper metal with too much sulfur and too much oxygen, too much for downstream direct wire production,” Allanore says.
Allanore lab’s new molten sulfide electrolysis method better handles trace metals and other impurities that come with the copper, allowing for separation of multiple elements at high purity from the same production process. “Therefore, we can rethink the manufacturing process of copper wires,” Allanore says.
“The essential part is about providing the sector — mining companies, existing smelting companies and existing copper producers — some data that show what happens on longer operations and at a larger scale,” Allanore says.
The International Copper Association conducted a Life Cycle Assessment that identified several areas where the copper industry can improve its environmental footprint. The study indicates the industry needs to continue reducing on-site sulfur dioxide emissions and to get its electricity from sources that are more environmentally friendly. Allanore’s project is relevant to both these issues. “If developed and deployed, it has the potential to decrease energy demand, operate entirely on renewable energy, and reduce sulfur dioxide emissions,” ICA technology director Stillman says. “In addition, it can separate unwanted impurities and recover valuable by-products from the concentrate. Right now, the technical evidence that is creating excitement is a small-scale proof-of-principle demonstration. It’s great that EERE has provided the needed initial funding to explore the potential. If the process works at larger scale, it could be the type of revolutionary approach that the industry is seeking.”
Allanore’s award is one of 24 early-stage, innovative technology projects receiving up to $35 million in support. It was announced by the U.S. Office of Energy Efficiency and Renewable Energy Advanced Manufacturing Office earlier this year.