Patients with a rare neuromuscular disorder and those with nerve damage tied to autoimmune disorders may share the same faulty synapses, neuroscientists at MIT’s Picower Institute for Learning and Memory report in the September issue of the American Journal of Human Genetics.
The work supports the notion that some muscle problems originate in nerve cells, not in the muscle itself.
The study identifies for the first time a presynaptic cause for a class of incurable neuromuscular diseases — known as congenital myasthenic syndromes — in which muscles weaken or waste away. The syndromes are tied to a genetic defect that can be inherited or occur spontaneously.
The study focused on individuals diagnosed with this rare neuromuscular disorder and others suffering from more common peripheral neuropathy, nerve damage that causes weakness, numbness, and pain associated with a host of causes from diabetes to autoimmune disorders.
“Some of the underlying mechanisms of peripheral neuropathies and neuromuscular disorders might be more closely linked than we previously thought,” says Troy Littleton, professor of biology in the departments of Brain and Cognitive Sciences and Biology at MIT. “This could mean that mutations in the same protein are causing an array of symptoms in the peripheral nervous system.”
The Littleton lab explores synapses--structures through which neurons communicate. Faulty synaptic connections have been linked to a wide range of neurological and psychiatric diseases, and Littleton hopes that shedding light on synapses’ structure and function will lead to new therapeutic approaches for these diseases.
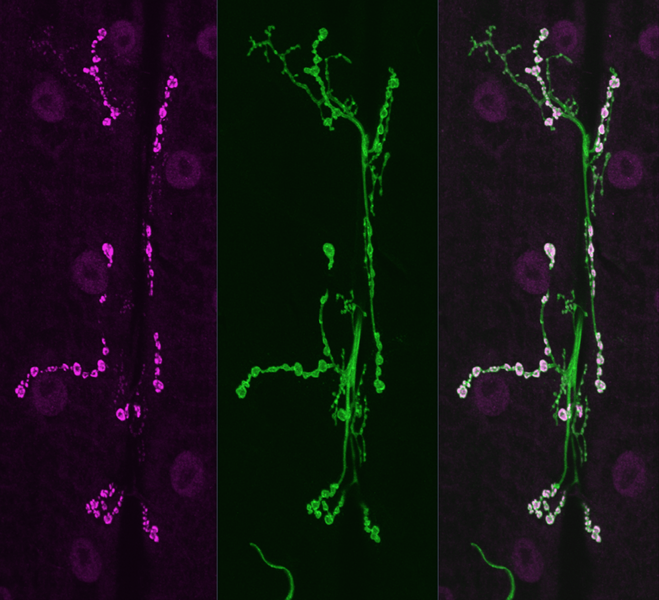
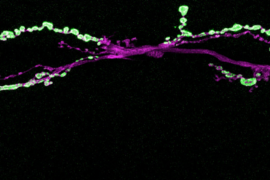
Teaming up with researchers at the University of Rochester Medical Center and the University of Miami Medical School who had studied the genetic makeup of individuals and families with congenital myasthenic syndrome, MIT research scientist Zhuo Guan and Littleton modeled the mutations for the first time in Drosophila, the common fruit fly, to explore exactly how the faulty genes altered synaptic transmission.
In the fly, the Littleton lab focused on synaptotagmin, a key protein in synaptic transmission that binds calcium and triggers the fusion of vesicles containing neurotransmitters that allow neurons to excite their targets. Humans have several synaptotagmin genes, including Synaptotagmin 2, which is principally found at synapses in the peripheral nervous system. The current study identified mutations in the calcium-binding domain of Synaptotagmin 2 as the key cause of a congenital myasthenic syndrome and peripheral neuropathy in several families in which the gene was mutated.
The finding that presynaptic genes can cause the disorder refocuses attention on how a defect in the motor nerve, not the muscle, may be contributing to the disease, Guan says. It also validates studies in animals that point to synaptotagmin as a calcium sensor that triggers neurotransmitter release.
“This study provides a new picture into potential genetic underpinnings of hereditary muscle weakness, and allows us to further dissect how these mutated synaptic proteins block the ability of peripheral nervous system motor axons to communicate," says Littleton.
“The fact that the same mutation can cause disease in humans and flies also indicates the mechanisms underlying the mutated protein are likely to be conserved, and we can use the fly model the figure out how the mutation actually disrupts the function of the protein,” Littleton adds. “This might allow us to figure out ways to treat these disorders as well.”