Whitehead Institute researchers have developed a novel method of removing potential cancer-causing genes during the reprogramming of skin cells from Parkinson's disease patients into an embryonic-stem-cell-like state. Scientists were then able to use the resulting induced pluripotent stem (iPS) cells to derive dopamine-producing neurons, the cell type that degenerates in Parkinson's disease patients.
The work marks the first time researchers have generated human iPS cells that have maintained their embryonic stem-cell-like properties after the removal of reprogramming genes. The findings are published in the March 6 edition of the journal Cell.
"Until this point, it was not completely clear that when you take out the reprogramming genes from human cells, the reprogrammed cells would actually maintain the iPS state and be self-perpetuating," says Frank Soldner, a postdoctoral researcher in Whitehead Member Rudolf Jaenisch's laboratory and co-author of the article.
Since August 2006, researchers have been reprogramming adult cells into iPS cells by using viruses to transfer four genes (Oct4, Sox2, c-Myc and Klf4) into the cells' DNA. Although necessary for reprogramming cells, these genes -- the known oncogene c-Myc in particular -- also have the potential to cause cancer. In addition, the four genes interact with approximately 3,000 other genes in the cell, which may change how the cell functions. Therefore, leaving the genes behind in successfully reprogrammed cells may cause unintended alterations that limit the cells' applicability for therapeutic use, for drug screens or to study disease in cell culture.
In the current method, Whitehead researchers used viruses to transfer the four reprogramming genes and a gene coding for the enzyme Cre into skin cells from Parkinson's disease patients. The reprogramming genes were bracketed by short DNA sequences, called loxP, which are recognized by the enzyme Cre.
After the skin cells were reprogrammed to iPS cells, the researchers introduced the Cre enzyme into the cells, which removed the DNA between the two loxP sites, thereby deleting the reprogramming genes from the cells. The result is a collection of iPS cells with genomes virtually identical to those of the Parkinson's disease patients from whom original skin cells came.
Removing the reprogramming genes is also important because of those genes' effect on an iPS cell's gene expression (a measure of which genes the cell is using and how much it's using those genes). When the researchers compared the gene expressions of human embryonic stem cells to iPS cells with and without the reprogramming factors, iPS cells without the reprogramming genes had a gene expression closer to human embryonic stem cells than to the same iPS cells that still contained the reprogramming genes.
"The reprogramming factors are known to bind to and affect the expression of 3,000 genes in the entire genome, so having artificial expression of those genes will change the cell's overall gene expression," Dirk Hockemeyer, who is also a co-author of the Cell article. "That's why the four reprogramming genes can mess up the system so much. From now on, it will be tough for researchers to leave the reprogramming genes in iPS cells."
Jaenisch, who is also a professor of biology at MIT and a member of the David H. Koch Institute for Integrative Cancer Research at MIT, says that the process to remove the reprogramming genes is very successful, when compared with earlier experiments. "Other labs have reprogrammed mouse cells and removed the reprogramming genes, but it was incredibly inefficient, and they couldn't get it to work in human cells," he says. "We have done it much more efficiently, in human cells, and made reprogrammed, gene-free cells."
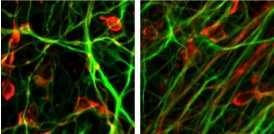
After removing the reprogramming genes, the Jaenisch researchers differentiated the cells from the Parkinson's disease patients into dopamine-producing nerve cells. In Parkinson's disease patients, these cells in the brain die or become impaired, causing such classic Parkinson's symptoms as tremors, slowed movement and balance problems.
Because the cells reside in the patients' brains, researchers cannot easily access them to investigate how the disease progresses at the cellular level, what kills the cells, or what might prevent cellular damage. Therefore, the ability to create patient-specific iPS cells, derive the dopamine-producing cells, and study those patient-specific cells in the lab could be a great advantage for Parkinson's disease researchers.
Although the initial results are extremely promising, Jaenisch acknowledges that the process is far from over. "The next step is to use these iPS-derived cells as disease models, and that's a high bar, a real challenge. I think a lot of work has to go into that."