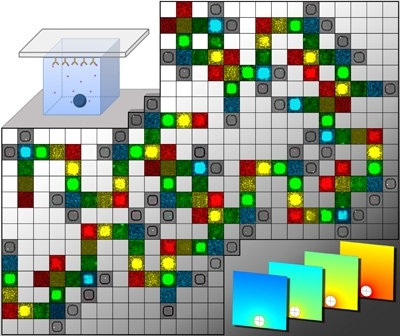
About 30 percent of Americans believe they have food allergies. However, the actual number is far smaller, closer to 5 percent, according to a recent study commissioned by the National Institute of Allergy and Infectious Diseases (NIAID). That’s due in large part to the unreliability of the skin test that doctors commonly use to test for food allergies.
MIT chemical engineer Christopher Love believes he has a better way to diagnose such allergies. His new technology, described in the June 7 issue of the journal Lab on a Chip, can analyze individual immune cells taken from patients, allowing for precise measurement of the cells’ response to allergens such as milk and peanuts.
Using this technology, doctors could one day diagnose food allergies with a simple blood test that would be faster and more reliable than current tests, says Love, an assistant professor of chemical engineering. “With a large number of diagnoses, it’s ambiguous,” he says. “A lot of times it’s almost circumstantial whether you’re allergic to one thing or another.”
Measuring single cells
In the United States, 6 to 8 percent of children under four, and 4 percent of people five or older, have at least one food allergy, according to the NIAID. Milk, peanuts, eggs and soy are among the most common allergens.
Food allergies occur when the body’s immune system mistakes a protein in food for something harmful. This triggers an allergic response that can include rashes, hives, difficulty breathing or gastrointestinal distress. Some allergies can provoke life-threatening anaphylactic shock, which requires immediate treatment.
Patients suspected of having food allergies usually undergo a skin test, which involves placing small quantities of potential allergens under the skin of the patient’s arm. If the patient’s blood has antibodies specific to that allergen, immune cells will release histamines that cause itching and redness in the spot where the allergen was placed.
Doctors can also perform blood tests that directly measure the presence of particular antibodies in the patients’ blood. However, one drawback to both of these tests is that the presence of antibodies to a particular allergen does not necessarily mean that the patient is allergic to that substance, leading to false positive results.
Love’s new technology, developed with funding from the Deshpande Center for Technological Innovation, the Dana Foundation and the NIAID, takes a different approach. Instead of detecting antibodies, his system screens the patient’s immune cells for small proteins known as cytokines. Immune cells such as T cells produce cytokines when an allergic response is initiated, attracting other cells to join in the response.
To perform the test, blood must be drawn from the patient, and white blood cells (which include T cells) are isolated from the sample.
The cells are exposed to a potential allergen and then placed into about 100,000 individual wells arranged in a lattice pattern on a soft rubber surface. Using a technique known as microengraving, the researchers make “prints” of the cytokines produced by each cell onto the surface of a glass slide. The amount of cytokine secreted by each individual cell can be precisely measured.
For food-allergy testing, the cytokines of most interest are IL4, IL5 and IL9.
Testing allergic reactions
The ability to measure individual immune cells’ cytokine production represents “a great advance,” says Amal Assa’ad, professor of pediatric immunology and allergy at the University of Cincinnati College of Medicine, who was not involved in the research. She adds, however, that clinical studies will be needed to demonstrate the ability to accurately diagnose food allergies. “Any test will have to be tested on multiple patients to see that it truly correlates with clinical allergy,” she says.
The “gold standard” for diagnosing a food allergy is to see what happens when the patient is given the food in question (in a controlled setting, to ensure safety), but that is not often done outside of allergy research clinics, says Assa’ad.
Love is now working with Dale Umetsu, professor of pediatric immunology at Children’s Hospital Boston, on a project they hope will pinpoint the relationship between cytokine activity and allergic reactions. In that study, children with milk allergies are being given small amounts of milk to desensitize their immune systems to the milk. Using the new technology, the team is tracking how the responses of the patients’ cells change as the patients undergo treatment.
MIT chemical engineer Christopher Love believes he has a better way to diagnose such allergies. His new technology, described in the June 7 issue of the journal Lab on a Chip, can analyze individual immune cells taken from patients, allowing for precise measurement of the cells’ response to allergens such as milk and peanuts.
Using this technology, doctors could one day diagnose food allergies with a simple blood test that would be faster and more reliable than current tests, says Love, an assistant professor of chemical engineering. “With a large number of diagnoses, it’s ambiguous,” he says. “A lot of times it’s almost circumstantial whether you’re allergic to one thing or another.”
Measuring single cells
In the United States, 6 to 8 percent of children under four, and 4 percent of people five or older, have at least one food allergy, according to the NIAID. Milk, peanuts, eggs and soy are among the most common allergens.
Food allergies occur when the body’s immune system mistakes a protein in food for something harmful. This triggers an allergic response that can include rashes, hives, difficulty breathing or gastrointestinal distress. Some allergies can provoke life-threatening anaphylactic shock, which requires immediate treatment.
Patients suspected of having food allergies usually undergo a skin test, which involves placing small quantities of potential allergens under the skin of the patient’s arm. If the patient’s blood has antibodies specific to that allergen, immune cells will release histamines that cause itching and redness in the spot where the allergen was placed.
Doctors can also perform blood tests that directly measure the presence of particular antibodies in the patients’ blood. However, one drawback to both of these tests is that the presence of antibodies to a particular allergen does not necessarily mean that the patient is allergic to that substance, leading to false positive results.
Love’s new technology, developed with funding from the Deshpande Center for Technological Innovation, the Dana Foundation and the NIAID, takes a different approach. Instead of detecting antibodies, his system screens the patient’s immune cells for small proteins known as cytokines. Immune cells such as T cells produce cytokines when an allergic response is initiated, attracting other cells to join in the response.
To perform the test, blood must be drawn from the patient, and white blood cells (which include T cells) are isolated from the sample.
The cells are exposed to a potential allergen and then placed into about 100,000 individual wells arranged in a lattice pattern on a soft rubber surface. Using a technique known as microengraving, the researchers make “prints” of the cytokines produced by each cell onto the surface of a glass slide. The amount of cytokine secreted by each individual cell can be precisely measured.
For food-allergy testing, the cytokines of most interest are IL4, IL5 and IL9.
Testing allergic reactions
The ability to measure individual immune cells’ cytokine production represents “a great advance,” says Amal Assa’ad, professor of pediatric immunology and allergy at the University of Cincinnati College of Medicine, who was not involved in the research. She adds, however, that clinical studies will be needed to demonstrate the ability to accurately diagnose food allergies. “Any test will have to be tested on multiple patients to see that it truly correlates with clinical allergy,” she says.
The “gold standard” for diagnosing a food allergy is to see what happens when the patient is given the food in question (in a controlled setting, to ensure safety), but that is not often done outside of allergy research clinics, says Assa’ad.
Love is now working with Dale Umetsu, professor of pediatric immunology at Children’s Hospital Boston, on a project they hope will pinpoint the relationship between cytokine activity and allergic reactions. In that study, children with milk allergies are being given small amounts of milk to desensitize their immune systems to the milk. Using the new technology, the team is tracking how the responses of the patients’ cells change as the patients undergo treatment.