MIT engineers have painted the most detailed portrait yet of how single cells from the immune system respond to vaccination.
The work, reported in the online edition of the Proceedings of the National Academy of Sciences the week of Nov. 3, could help researchers develop and test new vaccines for diseases including HIV, fungal infections and antibiotic-resistant bacterial infections.
"We're building a toolkit which we can use to look at how an immune response develops successfully. Then we aim to use that information for reverse engineering vaccines that would invoke that same type of response," said J. Christopher Love, assistant professor of chemical engineering and senior author of the paper.
Vaccines usually consist of an inactivated virus or bacterium that provokes B cells from the immune system to generate antibodies that attack the infectious agent.
Currently, the only way to test whether a vaccine has worked is to examine a patient's blood sample for the presence of antibodies. However, such tests do not offer a comprehensive picture of the immune system's ability to fight off infection, Love said.
"We don't know the diversity of antibodies generated, and we don't know how well they're responding to the pathogen. We don't know how poised the immune system is to respond to challenges it might face," he said.
His team's new approach generates information including the number of B cells present, whether they produce antibodies, the type of antibody they produce (for example, those that promote a long- or short-term response), the specificity (for a target like a protein from a virus or bacterium), and affinity (strength of binding to the target).
"This is the first time that it's possible to look at the diversity of antibody responses from primary cells, and measure a full set of their molecular characteristics, directly," Love said. "This really does give you a snapshot."
Currently, three different lab tests are needed to get all of that information, and one of the tests requires a very large number of cells. The new method works with as few as 100,000 cells (or the number in a small drop of blood).
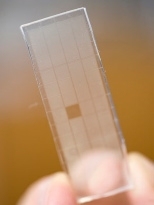
In their PNAS study, the researchers took B cells from mice that received a series of protein injections mimicking vaccination. They positioned the cells into individual containers, arranged in a dense lattice, molded into a soft rubber. Borrowing from an artistic engraving technique used for printmaking, the researchers use that array of cells to "print" the antibodies produced by the cells onto the surface of multiple identical glass slides.
Each of those slides is exposed to different concentrations of the protein used for the model vaccine, allowing the researchers to measure how strongly each antibody binds to the target. They can then map those results back to the original immune cell, pinpointing precisely which cells produced which antibodies and how strong the cell's response was.
In addition to vaccine development, the technique could be used to build a profile of a patient's immune system and its response to treatment for allergies, cancer or infectious diseases. "You could potentially track how the immune system is responding over time," Love said.
Lead author of the paper is Craig Story, former postdoctoral associate at the Whitehead Institute, now associate professor of biology at Gordon College. Other authors are Eliseo Papa, a graduate student in the Harvard-MIT Division of Health Sciences and Technology; Chih-Chi Andrew Hu, a post-doctoral fellow at the Whitehead Institute; Jehnna Ronan, a former Harvard undergraduate at the Whitehead Institute; and Hidde Ploegh, professor of biology and member of the Whitehead Institute.
The research was funded by the Broad Institute of MIT and Harvard, the National Institutes of Health, and the National Academies Keck Futures Initiative.
A version of this article appeared in MIT Tech Talk on November 5, 2008 (download PDF).