MIT biologists report a potential way to decrease the dose of chemotherapeutic agents needed to tackle cancer, a feat that would reduce these agents' toxic side effects.
What makes cancer cells unique is that they divide at a faster rate than ordinary cells, which makes them susceptible to the action of chemotherapeutic agents. But although chemotherapy is an effective treatment against fast-growing tumors, it is also associated with numerous toxic side effects because it is required at high doses to be effective.
Researchers from MIT's Center for Cancer Research (CCR) suggest a new approach to achieving the same response using a lower dose of chemotherapy, thereby limiting the harmful side effects of the drugs. Their approach involves making cancer cells even more sensitive to these agents.
In a paper to be published in the January 7, 2005 issue of Molecular Cell, a CCR research team led by Michael Yaffe, the Howard S. and Linda B. Stern Associate Professor of Biology, reports its results showing that blocking the function of the protein MAPKAP Kinase-2 increases the sensitivity of cancer cells to certain types of cancer treatment.
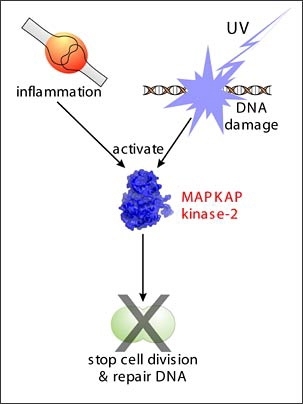
"MAPKAP Kinase-2 had been previously studied and was thought to be primarily involved in inflammation," explains Yaffe, who is also a member of the Department of Surgery at Beth Israel Deaconess Medical Center. "But our work shows that MAPKAP Kinase-2 also integrates DNA damage signaling responses and cell cycle arrest in mammalian cells."
"This result is particularly exciting as several drug companies are already developing MAPKAP Kinase-2 inhibitors for use in inflammation," said Yaffe. "Our hope is that we can use drugs already in development as anti-cancer agents."
Normal cells have a remarkable ability to sense when their DNA has been damaged and will repair the problem before continuing to copy their DNA and divide.
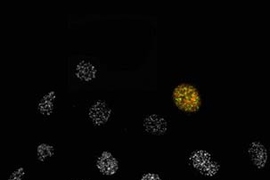
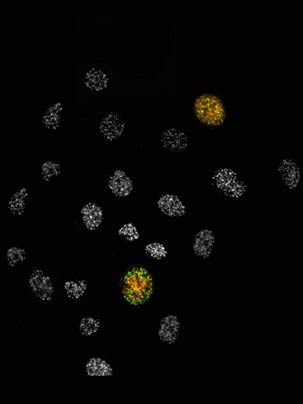
Using RNA interference (RNAi) to inhibit the activity of MAPKAP Kinase-2, Biology graduate student Isaac Manke showed that cells no longer sense DNA damage caused by ultraviolet light. Instead, he found the cells are more sensitive to the killing effects of ultraviolet light and also divide much faster.
"The discovery that MAPKAP Kinase-2 pathway functions to coordinate cell division and the DNA damage repair process is remarkable in its similarity to other pathways involved in the response to other types of DNA damage," said Manke.
In fact, clinical trials are underway to test the effectiveness of combining a drug that blocks the DNA damage response with chemotherapy to see if lower doses of chemotherapeutic agents may be used. The results of this MIT study suggest yet another new approach for improving a patient's response to chemotherapy.
Other MIT CCR researchers include postdoctoral fellows Daniel Lim and Mary Stewart, former postdoctoral fellow Anhco Nguyen and graduate student Andrew Elia.
Support for this work comes from the Jane Coffin Childs Foundation, the National Institutes of Health and the Burroughs-Wellcome Fund. Manke is supported by a Koch Graduate Fellowship.
A version of this article appeared in MIT Tech Talk on January 12, 2005 (download PDF).