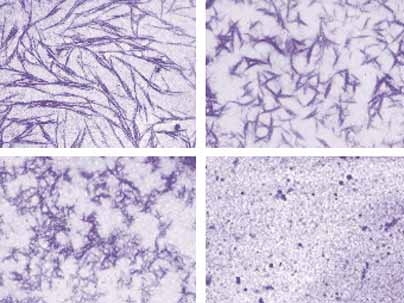
Amyloid fibers--those clumps of plaque-like proteins that clog up the brains of Alzheimer's patients--have perplexed scientists with their robust structures. Researchers don't yet have a way to assail these resilient molecules. Now a team from MIT and the Whitehead Institute for Biomedical Research reports that yeast may succeed where scientists have not.
In a study published online this week in the journal Science, the researchers describe a natural biological process by which yeast cells dismantle amyloid fibers.
"These proteins are remarkably stable," said Susan Lindquist, director of the Whitehead Institute, an MIT professor of biology and lead researcher on the project. "This is the first time that anyone has found anything that can catalytically take apart an amyloid fiber." Although the fibers are not necessarily the cause of Alzheimer's, they are associated with it and with many other neurological conditions.
The finding follows years of study on a yeast protein called Sup35, which helps cells translate genetic information into strings of amino acids, the building blocks of protein molecules. Sometimes Sup35 suddenly forms amyloid fibers similar to those found in Alzheimer's patients. In yeast, however, this doesn't kill the cell. Rather, it is part of the cell's normal biology, changing the types of proteins that the cell makes--changes that can sometimes be beneficial.
Previous research in the Lindquist lab described how a protein called Hsp104 seemed to affect Sup35's ability to form amyloid fibers. When a yeast cell contained either high amounts of Hsp104 or none at all, amyloid fibers never formed. But when Hsp104 levels were small, the fibers flourished.
While these types of relationships between chemicals aren't unheard of, the finding "was counterintuitive. Both high levels of Hsp104 and the absence of Hsp104 caused the same effect. That certainly made us want to figure out what was going on," said Lindquist.
In the new study, Lindquist and postdoctoral researcher James Shorter isolated Sup35 and Hsp104. They saw that small amounts of Hsp104 catalyzed the formation of amyloid fibers, but large levels of the protein actually caused the fibers to dissolve.
"Given their resilient structure, the fact that a protein can take apart these amyloids is remarkable," Lindquist said. "It has huge implications for our understanding of the protein folding process in amyloid-related conditions."
This research may also contribute to scientists' understanding of evolution. Prions, those infectious proteins implicated in conditions such as mad cow disease, are a subclass of amyloids. In yeast cells, Sup35 technically is a prion, although it is not toxic to the cell. Many researchers suspect that because prions have been so well conserved in yeast for hundreds of thousands of years, they must serve some sort of evolutionary purpose, and that's where Hsp104 comes in.
Hsp104 belongs to a class of proteins that sometimes is influenced by environmental factors. It is conceivable, Shorter explained, that a yeast cell in one type of environment can experience an abundance of Hsp104, which would then keep Sup35 from forming amyloid fibers in that cell. But put that cell in a different environment and the result may be a more moderate level of Hsp104 that would, in turn, create amyloid fibers in Sup35, changing how that protein functions and ultimately altering the cell's biology.
And because these changes could then be passed on to subsequent generations of cells, this would be an example of environment guiding the evolutionary process, the scientists noted.
"This is speculation that hasn't been demonstrated yet," Shorter said. "For obvious reasons it's hard to prove any evolutionary argument. But this paper is one indication that this might be the case."
The work was supported by a Charles A. Kind Trust postdoctoral fellowship and the National Institutes of Health.
A version of this article appeared in MIT Tech Talk on June 2, 2004 (download PDF).