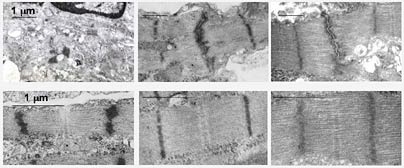
In a paper published this week in the online edition of the Proceedings of the National Academy of Sciences, engineers report creating a small swatch of heart tissue that displays many of the hallmarks of mature cardiac tissue, including regular contractions.
"We have been trying to engineer a patch of tissue that has the same properties as native heart tissue, or myocardium, that could be attached over injured myocardium," said Gordana Vunjak-Novakovic, a principal research scientist in the Harvard-MIT Division of Health Sciences and Technology (HST) who led the team of researchers.
"Think of it as a patch for a broken heart," she said.
The approach involves seeding cardiac cells, in this case from a rat, onto a 3-D polymer scaffold that slowly biodegrades as the cells develop into a full tissue. The cell/scaffolds, which are a little smaller than a dime and about the same thickness, are bathed in a medium that supplies nutrients and gases.
In a patent-pending technique, the researchers then apply electrical signals designed to mimic those in a native heart. They do so by essentially connecting the cell/scaffolds to a pacemaker. "Initially we had no idea if this would work. As it turns out, electrical stimulation was crucial for rapid assembly of functional tissue," said recent graduate Milica Radisic (Ph.D. 2004), who will be joining the faculty of the University of Toronto next year.
After only eight days of cultivation, single cells grew into a tissue "with a remarkable level of structural and functional organization," Vunjak-Novakovic said.
"The real advance here is we mimicked what the body does itself and got it to work," said Robert Langer, the Germeshausen Professor of Chemical and Biomedical Engineering and another member of the team.
"The greatest challenge," said Vunjak-Novakovic, "is to reproduce this with human cells, and test how all this works in the body."
Other authors of the PNAS article are Hyoungshin Park, an HST research engineer and co-first author with Radisic; Helen Shing and Frederick Schoen of Harvard Medical School; Thomas Consi, now on the faculty at the University of Wisconsin, Milwaukee; and Lisa Freed, an HST principal research scientist.
This work was funded by NASA, the NIH, and a Poitras fellowship.
A version of this article appeared in MIT Tech Talk on December 15, 2004 (download PDF).