After working at a software company for four years, MIT alumnus Andrew Dougherty MBA ’01 was itching to do something entrepreneurial in the energy industry.
Browsing the website of MIT’s $50K (now $100K) Entrepreneurship Competition, he found an exact match for his interests: an invention by MIT postdoc Javier García-Martínez that used nanotechnology to improve the efficiency of oil refining.
Refining of crude oil traditionally uses porous materials called zeolites as catalysts. When hydrocarbon compounds enter a zeolite’s micropores, they break down into transportation fuels and gas. But because of their pore size, the standard zeolites used for refining can’t diffuse the largest hydrocarbons.
García-Martínez had designed zeolites with pores that were 10 times larger. This technology would allow refineries to, for example, process more barrels or run heavier (and less expensive) crude oil feeds, leading to greater yields and profits.
“This was an innovative approach, so I emailed Javier, asking if he wanted help with his business plan — and he accepted,” Dougherty recalls.
The duo didn’t win the competition, but formed a business partnership to bring the technology to market — and eventually recruited into the fold MIT professor emeritus of chemical engineering Larry Evans, a seasoned entrepreneur.
In 2006, the three co-founded Rive Technology, a fast-growing startup that’s now commercializing García-Martínez’s invention. Headquartered in Boston with a research-and-development branch in Princeton, N.J., Rive has raised more than $67 million in venture capital and — as of 2010 — is partnered with W.R. Grace, a top global supplier for refining technology, to manufacture its first commercial product.
Two U.S. refineries have successfully trialed the technology. Last year, Rive demonstrated the technology’s effectiveness in a paper published in the journal Chemical Communications.
Rive’s ultimate aim, the co-founders say, is to use nanotechnology that targets hydrocarbons to transform oil refining — “the horsepower of the 20th century,” García-Martínez says — to a modern, efficient, and sustainable energy-production industry.
“We are going to be using hydrocarbons for many years to come, so we must use them more efficiently, reducing the amount of byproducts produced and increasing the quality of our fuels,” says García-Martínez, who is Rive’s chief scientist and a professor of chemistry and director of the Molecular Nanotechnology Lab at the University of Alicante in Spain.
Cruising the ‘molecular highway’
Most oil refineries today employ a process called fluid catalytic cracking, which uses specialized zeolites — called “Y Type,” and usually synthesized into a fine powder — as catalysts.
These zeolites have micropores — measuring about 1 nanometer in diameter — that allow only small hydrocarbon compounds to enter. Strong acids then break, or “crack,” the hydrocarbons into smaller fragments, which constitute desirable products such as gasoline, kerosene, and diesel fuel.
At MIT, García-Martínez learned that industry and academia had spent decades, and millions of dollars, trying to increase the size of zeolite pores while maintaining molecular structural integrity.
So while working as a postdoc at MIT from 2001 to 2003, he developed a method of expanding the zeolite micropores into mesopores, which measure between 7 and 10 nanometers in diameter — the ideal range for producing gasoline, García-Martínez says. To do so, he mixed zeolites with an alkaline solution and added a temporary surfactant, which forms small structures that the zeolites reconstruct around. The surfactant then burns off, leaving zeolites with a high number of mesopores.
“These mesopores act as highways that connect the smaller micropores, significantly improving the molecular traffic inside zeolites,” García-Martínez explains. Rive calls these broad channels “molecular highways,” for which its commercial product is named.
The product, a platform for this technology, has proven effective in increasing refining yields and profits. At the annual meeting of the American Fuel and Petrochemical Manufacturers in March, for instance, Rive presented a paper showing that the second generation of its “Molecular Highway” technology generated a value uplift of $2.50 per barrel at a Texas refinery.
“Our process is both inexpensive — a must in an industry that deals with thousands of tons of catalyst per day — and very precise, in the way mesoporosity is incorporated to the zeolite without compromising its key properties: activity and stability,” García-Martínez says. “Also, it is very convenient for the refiner, because we are not introducing a whole new zeolite, but only incorporating ‘controlled mesoporosity’ to the zeolite the refiner has been using for years.”
The technology, García-Martínez says, can be used in a wide range of applications, such as water and air treatment and converting waste and biomass to useful materials and energy. Results of a 2012 study, sponsored by the U.S. Department of Energy, for instance, suggest the technology can reduce the energy used in separation of propane from propylene by 70 percent.
Refining a business
In developing his technology, García-Martínez initially had no commercial aspirations. But enough time at MIT — with access to business competitions, mentors, and the Technology Licensing Office — changed his mind.
“At MIT, there is a method, a well-established and successful way to transfer technology from the lab to the marketplace,” he says. “Seasoned professionals helped me protect my technology, create my team, and build my network with potential investors, lawyers, and mentors.”
One member of this network was Evans, who had co-founded Aspen Technology — the software company where Dougherty worked — in the 1980s. Among other things, Evans helped Dougherty and García-Martínez connect with venture capitalists. (Evans was Rive’s CEO until he stepped down in 2012.)
During Rive’s expansion, Dougherty became one of the company’s key business strategists. Early on, he was a “business generalist,” helping hire, fundraise, and set up the R&D lab, among other things. Two years ago, he became vice president of sales and marketing. He acknowledges that his days at the MIT Sloan School of Management and the Martin Trust Center for MIT Entrepreneurship had taught him “the nuts and bolts” of building a business.
“Certainly in the early days of Rive, I was leaning heavily on what I learned at Sloan and the entrepreneurship center, such as fundraising, constructing cash-flow models, and building a business plan that could withstand the scrutiny of the investment community,” he says.
One strategy Dougherty suggested early on was a “partnering model”: Instead of manufacturing zeolites, Rive needed to partner with other companies that already manufactured zeolites — such as W.R. Grace. Additionally, the company follows a strategy of staying involved in the sales and deployment of the product
“We made a conscious decision to be very engaged in the end use of technology,” Dougherty says. “We own the customer dialogue, own the pricing of technology. In that way, we’re driving the sale of this technology in the marketplace.”
Ultimately, García-Martínez believes Rive’s technology is demonstrative of how nanotechnology — and specifically, “controlled porosity” — can improve global energy consumption. “I am personally convinced that nanotechnology in general, and materials with controlled porosity in particular, hold the promise to solve some of our most pressing challenges, such as cleaner energy production, mitigating climate change, and better water and air quality,” he says.
Startup Rive Technology is commercializing an MIT-developed invention that improves catalysts used in oil refining, leading to greater yields.
Publication Date:
Caption:
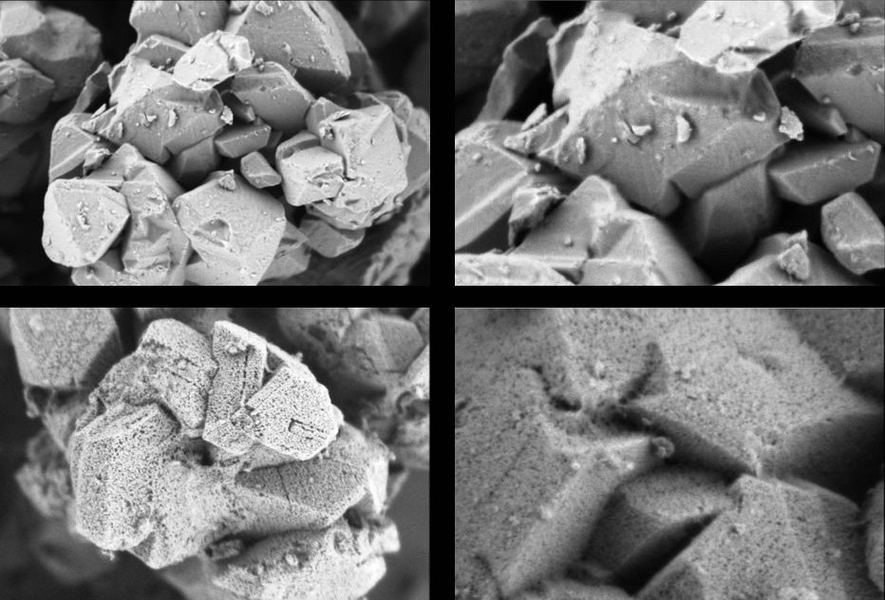
The above image shows (top) two micrographs at different magnifications of a standard "Y Type" zeolite with micropores, and (bottom) two micrographs at different magnifications of the same zeolite that has been given mesopores (much larger holes) through Rive Technology's Molecular Highway product.
Credits:
Image courtesy of Rive Technology