CAMBRIDGE, Mass.--In work that could aid the development of better batteries for products from laptop computers to electric cars, an MIT engineer and colleagues have taken the first images of individual atoms of lithium, a key ingredient in state-of-the-art rechargeable batteries. Only atoms of hydrogen and helium are smaller and lighter than those of lithium.
"The atomic resolution of lithium atoms is a novel and significant achievement, with implications for better understanding not only of lithium ion battery materials but of many other electroceramic materials as well," said Yang Shao-Horn, an assistant professor in MIT's Department of Mechanical Engineering and lead author of a paper on the work to appear in the June 15 issue of Nature Materials.
Her coauthors are Laurence Croguennec, Claude Delmas, E. Chris Nelson, and Michael O'Keefe. Croguennec and Delmas are from the Institute de Chimie de la Matière Condensée de Bordeaux-CNRS and the Ecole National Supèrieure de Chimie et Physique de Bordeaux at the University of Bordeaux I. Nelson and O'Keefe are from Lawrence Berkeley National Laboratory.
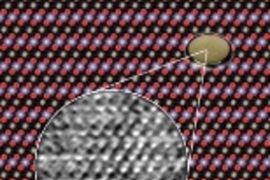
The team used a transmission electron microscope--the One Angstrom Microscope at the Department of Energy's National Center for Electron Microscopy at Berkeley Lab--to simultaneously resolve columns of lithium, cobalt, and oxygen atoms in the compound lithium cobalt oxide (LiCoO2).
LiCoO2 is commonly used in the positive electrodes of lithium rechargeable batteries, whose operation is based on reversible insertion and removal of lithium ions to and from their positive and negative electrodes. Widely used in laptop computers, digital cameras, and many other devices, lithium ion batteries store more energy for their weight, operate at a higher voltage, and hold a charge much longer than other rechargeable batteries.
To improve their performance will require understanding how the atoms in the electrode materials--and the vacancies left by moving ions--are arranged in 3-D on the atomic scale. Although the structure of LiCoO2 is known theoretically and has been confirmed with other techniques, lithium ions have never been seen by these techniques, nor have they been seen in previous attempts to image LiCoO2 with electron microscopy.
Thus when Shao-Horn approached microscopist O'Keefe in the summer of 2001 to ask whether it would be possible to image lithium ions in the material with a high-resolution transmission electron microscope (TEM), his first answer was, "I don't think so."
But using a computer simulation program developed by O'Keefe, the researchers determined such essential information as what resolution the One Angstrom Microscope had to achieve to see anything at all, and at what resolution all the different kinds of atoms should be sharpest.
The program showed that in a sample of the right thickness, lithium ions should become visible at 1 angstrom (an angstrom, symbolized Ã…, is a ten-billionth of a meter). And at 0.8 Ã… all three kinds of atoms should be clearly visible--a resolution that was, indeed, just possible to reach with the One Angstrom Microscope.
O'Keefe says the computer program "showed that the columns of oxygen atoms should look bright and sharp-edged, the cobalts should be fuzzy, and the lithium should be small, weak, and look a little stretched."
In 2002, working with Berkeley Lab's Nelson to master the operation of the One Angstrom Microscope, Shao-Horn obtained many series of 20 differently focused images of individual crystals from a LiCoO2 powder sample. The sample was prepared and well characterized by conventional X-ray diffraction in collaboration with her colleagues at the University of Bordeaux I.
Using a second computer program and their measurements of the microscope parameters, the researchers took the experimental images and worked backwards to produce a representation of the electron wave leaving the exit surface of the specimen. They then used the program to produce an image from a small area of a thin edge of a LiCoO2 crystal.
Says O'Keefe, "We have shown that the range of the One Angstrom Microscope, and mid-voltage microscopes like it, can be extended all the way from heavy atoms down through oxygen, nitrogen, and carbon to the lightest metal--in fact, except for helium and hydrogen, the lightest atoms of all."