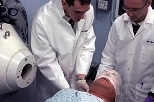
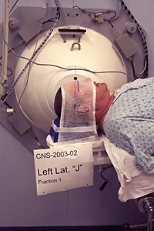
Researchers from the Beth Israel Deaconess Medical Center and MIT have begun advanced clinical trials of a cancer treatment that could selectively target malignant tissue while reducing the likelihood of injury to healthy tissue.
The trials are open to patients suffering from glioblastoma multiforme (GBM, a virulent form of brain cancer), melanoma that has spread to the central nervous system, and melanoma in the extremities.
The trials are the first to use a new MIT facility dedicated to the treatment. The facility, the only one in the United States, is considered the best of its kind in the world.
All three cancers are characterized by malignant cells that have proven difficult to eradicate with conventional forms of radiation.
"For these cancers, conventional radiation therapy or surgery is not able to destroy all the tumor cells without unacceptable damage to healthy tissues," said Professor Otto K. Harling of the Department of Nuclear Engineering. The new treatment, known as neutron capture therapy (NCT), "has the potential to destroy tumor cells while sparing adjacent healthy cells."
Patients who are candidates for NCT clinical trials may have already undergone surgery for removal of as much of the tumor as possible.
The patient is given an intravenous dose of a compound containing boron-10, known as the "capture drug." He or she is then placed on a couch for positioning in the epithermal neutron beam, which is specially designed to penetrate at least halfway into the brain to reach deep-seated tumors. A device called a patient collimator--reminiscent of the device used for dental X-rays--directs the beam toward the desired region. To be effective, the neutron beam must be very pure to deliver the maximum possible dose to the tumor.
Five years ago, the exposure period lasted a few hours. Today, irradiations can be completed in a few minutes, minimizing discomfort. Delivery of the boron compound followed by irradiation in the neutron beam is broken into two sessions, delivered on consecutive days.
Dr. Paul M. Busse of Beth Israel Deaconess and Harvard Medical School calls NCT promising. "We are encouraged by our clinical results thus far and also by those of our colleagues in Europe and Japan," he said. "The new [facility] at MIT is second to none as is the research team assembled at MIT and Harvard to do this work."
Harling directed the design and construction of the new Fission Converter Epithermal Neutron Irradiation Facility. Housed in MIT's research reactor, it replaces a facility in the same location that had been used for 15 years. Busse is director of the clinical aspects of the research. Robert G. Zamenhof (MIT Ph.D. 1977), currently at Beth Israel Deaconess Medical Center and Harvard Medical School, heads the project's medical physics component.
In 1999 the team concluded a Phase I clinical trial of NCT for the cancers that are the focus of the current work. Phase I trials aim to determine the safety of the technique and involve a process of gradual dose increase to determine the maximum safe dose of radiation.
One of the current trials again focuses on GBM and on melanoma in the central nervous system. This trial combines elements of both Phase I and II trials. Phase II trials are designed to determine the benefits of the experimental treatment at the dose levels established in Phase I. A separate Phase II trial is under way for melanoma on the extremities. These trials are sponsored by the National Cancer Institute and the National Institutes of Health.
NCT involves a drug and irradiation with neutrons. It is unique because it combines a biological and physical targeting of dose. The drug, which contains boron-10, is designed to concentrate preferentially in tumor cells. The patient is then exposed to a specially designed beam of "epithermal" neutrons.
The neutrons in turn interact with the boron, which releases subatomic particles that kill the tumor cells. Because these particles travel only short distances--approximately the length of a cell--the researchers expect that they will primarily damage the tumor cells and not healthy brain cells nearby.
Harling noted that patients treated with NCT might only have to be irradiated over one or two days, as compared to the 30 days typical for conventional treatments.
MIT has conducted research on this approach to radiation therapy since the 1950s. Harling himself has been working on it since 1985.
"As I got older in my professional career, I wanted to do something that was closer to helping people," he said. "I felt I could make a contribution in this area since I've spent many years working with neutrons, so I helped put together a team, we got a hospital involved, and I'm still at it and cautiously optimistic that we will succeed in developing a better cancer therapy."
The new NCT facility was funded by the Department of Energy and MIT. Additional information on the clinical trials and research can be found at http://www.bnct.org.
Who's eligible
To participate in the cancer trials, a patient must be at least 18 years old, have been diagnosed with glioblastoma multiforme, a primary brain tumor, or metastatic melanoma in the brain; have had no radiation therapy, and be able to walk and perform basic activities of daily living. A National Cancer Institute grant covers expenses related to the treatment. For additional information, contact cancer research nurse Jody Kaplan at 617-667-4679.
A version of this article appeared in MIT Tech Talk on May 21, 2003.