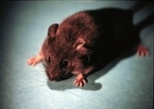
Tetley is no ordinary mouse. And it's not just because he's a clone. Tetley is special because he was created using a new technology that researchers say has produced the most efficient results to date for cloning mice.
He is also the first mouse clone whose genetic material was modified in the laboratory before cloning. The technology used to create Tetley, say researchers, will have a major impact on improving the efficiency of cloning in general.
In the February issue of Nature Genetics, Whitehead Institute and University of Hawaii researchers led by Professor of Biology and Whitehead member Rudolf Jaenisch reported that they have successfully used embryonic stem cells to clone mice with the highest efficiency to date. Their study comparing clones generated by two different donor strains suggests that the genetic makeup of donor cells plays a key role in determining the viability of clones.
In addition, the scientists show for the first time that it is possible to modify the genetic material in embryonic cells before using them to clone new animals. The scientists inserted a gene derived from the tetracycline (tet) receptor into an embryonic stem cell and then transplanted the modified genome into an egg cell whose genetic material had been removed. The result was Tetley: a transgenic clone who now carries the tet gene. The new technology will improve cloning efficiency by providing researchers the genetic tools needed to understand why so few clones survive to term and grow into healthy adults.
"Our technology will help scientists tackle one of the major challenges of mammalian cloning -- the low numbers of viable clones," said Professor Jaenisch. "Most clones die during gestation or soon after birth, and there are many possible reasons for this, including genetic makeup of the donor, the cell-cycle stage of the donor cell, loss of genetic information or the inability of the egg cell to reprogram the donor cell nucleus."
Assessing the impact of these factors on cloning efficiency has been difficult because most cloning experiments have used somatic cells -- adult cells that have specialized to perform specific functions in the body. Somatic cells have a limited lifespan, and they are difficult to manipulate genetically. As a result, they make poor tools for studying the genetic basis of cloning efficiency.
The technology of cloning using embryonic stem cells removes that obstacle, allowing researchers to study the various parameters that affect cloning efficiency, said Professor Jaenisch. Embryonic stem cells normally direct the development of an entire animal. They can grow indefinitely in culture and can also be genetically manipulated to carry extra genes or knockout genes under study. Thus they provide researchers the perfect genetic tool to study cloning.
Although the federal government and most scientists believe human cloning to be unethical, experts agree that improving cloning technology could have real benefits in agriculture and animal husbandry, as well as in biomedical research designed to decipher the mechanisms of disease. The mouse, in particular, is considered the best mammalian model system to study the genetic basis of human disease.
RESEARCH RESULTS
In the Nature Genetics study, the Jaenisch lab members and their collaborators at the University of Hawaii produced mouse embryos by transplanting genetic material from embryonic stem cells of two different strains of donor mice into enucleated egg cells. They then implanted these embryos into surrogate mice. After the transfer, seven of the 34 embryos -- 21percent -- from the first strain developed to term and grew into healthy adults. By contrast, only eight of 76 embryos made from the second, more inbred strain developed to term. All eight died within 24 hours after birth, demonstrating that genetic makeup of donor cells may be a key to survival of clones.
"Embryonic stem cells from outbred mice created clones with better efficiency than embryonic stem cells from inbred mice. Thus, the ability to generate viable clones from ES cells might be correlated with their genetic diversity," said Dr. William Rideout, a postdoctoral fellow in the Jaenisch lab.
Scientists also found that the survival rate of transferred embryos was higher with cloning using embryonic stem cells rather than somatic cells, probably because embryonic stem cells need less reprogramming of gene expression (i.e., turning back of the developmental clock) than somatic cells.
This and other information from ES cell cloning will help researchers maximize cloning efficiency.
Finally, the study points to a new way of making transgenic animals that could be faster and more efficient, say scientists. "Transgenic science is an important research tool because it allows us to insert mutations in a gene and study how it affects the whole animal. If we know which gene is mutated in a particular human disease, we can develop mouse models with the same mutation," said Dr. Rideout.
Today, mouse models of diseases such as epilepsy, colon cancer, hypertension and diabetes are providing new insights into the genetic basis of these diseases, but transgenic animals are cumbersome to make, and it takes three to nine months for scientists to create a new strain. Scientists say that using targeted ES cells to clone animals will cut by one-third the time it takes to create mouse models.
The study was funded in part by the National Institutes of Health.
Other Whitehead authors of the paper in addition to Professor Jaenisch are postdoctoral fellows Rideout and Anton Wutz; postdoctoral associate Laurie Jackson-Grusby; Kevin Eggan, a graduate student in biology; and research specialist Jessica Dausman.
A version of this article appeared in MIT Tech Talk on February 9, 2000.