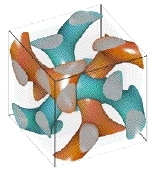
CAMBRIDGE, Mass. -- It looks like something out of an M.C. Escher illustration: a three-pronged vine intertwined with a mirror twin in a seemingly endless matrix. The two twirl around each other to create a dense scaffold, but never touch.
In a paper that will appear in the November 26 issue of Science, a Massachusetts Institute of Technology researcher studied two materials that self-assemble into the twisting shape called the double gyroid. Subjecting the materials to a one-step, room-temperature oxidation process converts them into nanoporous or nanorelief ceramic films with a myriad of potential uses.
Nanoporous structures have tiny holes. Nanorelief structures are like miniature jungle gyms. Their interconnected struts form a three-dimensional structure that is mostly air. In this case, the struts are only 300 Angstroms in diameter, or about one-thousandth the width of a human hair.
As a ceramic, the films can be subjected to temperatures up to 400 degrees Celsius -- much higher than traditional polymers can withstand. It also is potentially solvent-resistant, which expands its applications. Normally, "ceramics are powders and binders. They don't self-assemble into this shape," said Vanessa Chan, the paper's first author and a recent MIT Ph.D. in materials science and engineering.
Creating new inorganic materials with ordered pores or struts is an area of increasing interest to industry, she said, because of the materials' potential applications as selective membranes, next-generation catalysts and photonic materials.
INTERTWINED PATHWAYS
Chan chose to work with materials possessing the intriguing double gyroid shape on a nanometer scale because the intricate and unusual double-strand matrix cannot be made through more conventional processing techniques.
Researchers have found that by carefully controlling the molecular weight and volume fraction of the block copolymers (a type of polymer made up of two or more different monomer units linked together in blocks), nanostructures with highly ordered and complex pore structures can be produced. These tiny structures are being eyed as potential components in integrated circuits and magnetic storage media, Chan said.
Comprised of an inorganic-containing block and a purely hydrocarbon block, two materials were synthesized that form two different structures: one where the inorganic block is dominant and one where the organic block is dominant. Through oxidation, the hydrocarbon block can be removed and the inorganic-containing block converted to a ceramic.
With these two structures, researchers can create a ceramic matrix interpenetrated with connected, ordered, tortuous pores or an open structure comprised of intricate 3D ceramic strut networks. The pore size is in an important mid-range where no comparable commercial filtration materials currently exist.
Among other things, the ceramic film might be good for as high-temperature membranes for chemical and biological separations. The multitude of redundant paths means that flow is protected against local clogging.
"I think there will be a lot of interest in applying this material and process to commercial applications, when you think of its simplicity," said Chan, who with her colleagues has filed for a patent on the nanoporous and nanorelief ceramic films.
THE IMAGINARY BECOMES REAL
The double gyroid structure was discovered in a block copolymer system in Prof. Edwin L. Thomas's group at MIT in 1994. However, mathematicians had created a theoretical version 30 years earlier.
Structural identification of this geometry was a challenge because researchers viewed the complex, three-dimensional gyroid structures as two-dimensional projections in microscopy. To understand the projections, co-author James Hoffman of the Mathematical Science Research Institute in Berkeley, Calif., provided simulations of the theoretical surface that matched the real-life MIT creation.
"Sometimes mathematicians study interface structures. Then technologists come along and say what the structures are good for," said Thomas, Morris Cohen Professor of Materials Science and Engineering at MIT and one of the article's co-authors.
To Chan, the fact that she has a real-life example of an imaginary structure created by a mathematician is amazing. "You have an equation that defines a surface, and here the same structure exists in nature," she said.
This work was a result of collaboration among chemists, mathematicians and material scientists from MIT, IBM, the University of California at Berkeley and the University of Athens. Victor Lee of IBM and Hermis Iatrou, Apostolos Avgeropoulos and Nikos Hadjichristidis of the University of Athens chemistry department synthesized the polymeric materials. Chan, under the guidance of Robert Miller of IBM and Edwin Thomas of MIT developed the process to produce the ceramic nanostructures.
This work was funded by the Air Force Office of Scientific Research and the National Science Foundation (NSF) Center for Polymer Interfaces and Macromolecular Assemblies at IBM. Chan was funded by NSF and IBM Graduate Research Fellowships.