Catherine Drennan says nothing in her job thrills her more than the process of discovery. But Drennan, a professor of biology and chemistry, is not referring to her landmark research on protein structures that could play a major role in reducing the world’s waste carbons.
“Really the most exciting thing for me is watching my students ask good questions, problem-solve, and then do something spectacular with what they’ve learned,” she says.
For Drennan, research and teaching are complementary passions, both flowing from a deep sense of “moral responsibility.” Everyone, she says, “should do something, based on their skill set, to make some kind of contribution.”
Drennan’s own research portfolio attests to this sense of mission. Since her arrival at MIT 20 years ago, she has focused on characterizing and harnessing metal-containing enzymes that catalyze complex chemical reactions, including those that break down carbon compounds.
She got her start in the field as a graduate student at the University of Michigan, where she became captivated by vitamin B12. This very large vitamin contains cobalt and is vital for amino acid metabolism, the proper formation of the spinal cord, and prevention of certain kinds of anemia. Bound to proteins in food, B12 is released during digestion.
“Back then, people were suggesting how B12-dependent enzymatic reactions worked, and I wondered how they could be right if they didn’t know what B12-dependent enzymes looked like,” she recalls. “I realized I needed to figure out how B12 is bound to protein to really understand what was going on.”
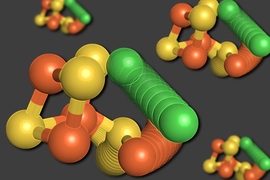
Drennan seized on X-ray crystallography as a way to visualize molecular structures. Using this technique, which involves bouncing X-ray beams off a crystallized sample of a protein of interest, she figured out how vitamin B12 is bound to a protein molecule.
“No one had previously been successful using this method to obtain a B12-bound protein structure, which turned out to be gorgeous, with a protein fold surrounding a novel configuration of the cofactor,” says Drennan.
Carbon-loving microbes show the way
These studies of B12 led directly to Drennan’s one-carbon work. “Metallocofactors such as B12 are important not just medically, but in environmental processes,” she says. “Many microbes that live on carbon monoxide, carbon dioxide, or methane — eating carbon waste or transforming carbon — use metal-containing enzymes in their metabolic pathways, and it seemed like a natural extension to investigate them.”
Some of Drennan’s earliest work in this area, dating from the early 2000s, revealed a cluster of iron, nickel, and sulfur atoms at the center of the enzyme carbon monoxide dehydrogenase (CODH). This so-called C-cluster serves hungry microbes, allowing them to “eat” carbon monoxide and carbon dioxide.
Recent experiments by Drennan analyzing the structure of the C-cluster-containing enzyme CODH showed that in response to oxygen, it can change configurations, with sulfur, iron, and nickel atoms cartwheeling into different positions. Scientists looking for new avenues to reduce greenhouse gases took note of this discovery. CODH, suggested Drennan, might prove an effective tool for converting waste carbon dioxide into a less environmentally destructive compound, such as acetate, which might also be used for industrial purposes.
Drennan has also been investigating the biochemical pathways by which microbes break down hydrocarbon byproducts of crude oil production, such as toluene, an environmental pollutant.
“It’s really hard chemistry, but we’d like to put together a family of enzymes to work on all kinds of hydrocarbons, which would give us a lot of potential for cleaning up a range of oil spills,” she says.
The threat of climate change has increasingly galvanized Drennan’s research, propelling her toward new targets. A 2017 study she co-authored in Science detailed a previously unknown enzyme pathway in ocean microbes that leads to the production of methane, a formidable greenhouse gas: “I’m worried the ocean will make a lot more methane as the world warms,” she says.
Drennan hopes her work may soon help to reduce the planet’s greenhouse gas burden. Commercial firms have begun using the enzyme pathways that she studies, in one instance employing a proprietary microbe to capture carbon dioxide produced during steel production — before it is released into the atmosphere — and convert it into ethanol.
“Reengineering microbes so that enzymes take not just a little, but a lot of carbon dioxide out of the environment — this is an area I’m very excited about,” says Drennan.
Creating a meaningful life in the sciences
At MIT, she has found an increasingly warm welcome for her efforts to address the climate challenge.
“There’s been a shift in the past decade or so, with more students focused on research that allows us to fuel the planet without destroying it,” she says.
In Drennan’s lab, a postdoc, Mary Andorfer, and a rising junior, Phoebe Li, are currently working to inhibit an enzyme present in an oil-consuming microbe whose unfortunate residence in refinery pipes leads to erosion and spills. “They are really excited about this research from the environmental perspective and even made a video about their microorganism,” says Drennan.
Drennan delights in this kind of enthusiasm for science. In high school, she thought chemistry was dry and dull, with no relevance to real-world problems. It wasn’t until college that she “saw chemistry as cool.”
The deeper she delved into the properties and processes of biological organisms, the more possibilities she found. X-ray crystallography offered a perfect platform for exploration. “Oh, what fun to tell the story about a three-dimensional structure — why it is interesting, what it does based on its form,” says Drennan.
The elements that excite Drennan about research in structural biology — capturing stunning images, discerning connections among biological systems, and telling stories — come into play in her teaching. In 2006, she received a $1 million grant from the Howard Hughes Medical Institute (HHMI) for her educational initiatives that use inventive visual tools to engage undergraduates in chemistry and biology. She is both an HHMI investigator and an HHMI professor, recognition of her parallel accomplishments in research and teaching, as well as a 2015 MacVicar Faculty Fellow for her sustained contribution to the education of undergraduates at MIT.
Drennan attempts to reach MIT students early. She taught introductory chemistry classes from 1999 to 2014, and in fall 2018 taught her first introductory biology class.
“I see a lot of undergraduates majoring in computer science, and I want to convince them of the value of these disciplines,” she says. “I tell them they will need chemistry and biology fundamentals to solve important problems someday.”
Drennan happily migrates among many disciplines, learning as she goes. It’s a lesson she hopes her students will absorb. “I want them to visualize the world of science and show what they can do,” she says. “Research takes you in different directions, and we need to bring the way we teach more in line with our research.”
She has high expectations for her students. “They’ll go out in the world as great teachers and researchers,” Drennan says. “But it’s most important that they be good human beings, taking care of other people, asking what they can do to make the world a better place.”
This article appears in the Spring 2019 issue of Energy Futures, the magazine of the MIT Energy Initiative.