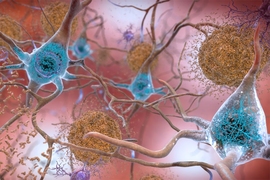
Tens of millions of people worldwide suffer from neurodegenerative diseases such as Alzheimer’s, Parkinson’s, multiple sclerosis, and Lou Gehrig’s disease — but no effective treatments exist for these conditions.
Research Scientist Anna Jagielska of the MIT Department of Materials Science and Engineering thinks repairing the myelin wrapping around axons is key to preserving neurological function and slowing or stopping neurodegeneration. Her team, with support from the MIT Deshpande Center for Technological Innovation, the U.S. Department of Defense, Sanofi-Genzyme, and others, is developing artificial axons using advanced 3D printing, in the hopes of accelerating the discovery of drugs that stimulate myelin repair.
Q: What are the key barriers to developing drugs to treat neurodegenerative diseases?
A: The current lack of a predictive disease model and drug discovery tools results in more than 90 percent of neurological drug candidates failing in clinical trials. Having a predictive tool would save pharmaceutical companies time and costs in what is a long, multiyear process of drug development.
Our tools focus on myelin, and many neurological diseases are associated in some ways with damage or disorders of myelin, the protective sheath around axons. Each neuron has one long, thin fiber called an axon that transmits electrical impulses throughout the nervous system so we can move our limbs, see, and breathe. When the myelin coating around axons is damaged, a process known as demyelination, nerve conduction slows or is lost and axons can die. This can impact motor and cognitive functions, and lead to loss of vision and permanent disabilities. In many of these diseases, the body does not regenerate myelin sufficiently on its own. However, if a drug could stimulate the body to generate new myelin sheaths, a process known as remyelination, this could protect axons from dying and preserve their neurological function. We are developing artificial axons that mimic an environment where myelin grows and wraps around these axons as if they were in the brain. This tool would allow researchers to see how effectively different drugs spur the growth of myelin.
Q: How can artificial axons be potentially transformative for drug discovery?
A: Artificial axons fill an unmet need, providing the right tools to begin to address these neurological diseases. By supplying a sufficiently accurate representation of the neural environments for each of these illnesses, we’re hoping to help develop therapies that may alleviate them. Finding drugs that restore myelin would help slow the progression of illnesses like multiple sclerosis, which is marked by successive bouts of demyelination that lead to a progressive loss of nervous system function.
The early-stage development of such drugs is where this technology can be most helpful. The artificial axons mimic compliant brain cells and facilitate direct quantification of myelination. The 3D-printed platform balances the complexity of neurons’ biofidelic features with the simplicity of engineered polymer arrays to watch and quantify oligodendrocytes, the myelinating cells of the brain, as they grow, mature, and wrap myelin around the artificial axons, in the same way they would do so in the brain.
Our platform has many advantages over current tools. Traditional flat-tissue culture dishes made of hard, stiff plastic provide the wrong environment for neural cells, possibly altering cells’ responses to drugs as compared to how cells would respond in the body. Moreover, it is not possible to study myelination in these flat dishes, because this process requires the presence of three-dimensional, axon-like structures. The artificial axons, on the other hand, mimic real axons’ low mechanical stiffness, which is six orders of magnitude less stiff than plastic dishes, as well as axons’ geometrical properties, down to orders of micrometers. The artificial axons are also drug-agnostic, meaning they allow a variety of compounds to be tested on it. The platform is highly tunable. Artificial axons can be printed with different shapes, diameters, densities, mechanical properties, and surface ligands to model specific diseases.
Our format is compatible with pharmaceutical setups for drug screening. We have improved fabrication throughput to produce samples with high reproducibility in a short period of time, a 96-well plate within minutes.
Q: How is the composition of your team especially suited to creating these tools for the drug discovery process?
A: Our group in the Van Vliet Laboratory for Material Chemomechanics is diverse in expertise. We have people with experience in both cell biology and materials development. We also develop tools to study and characterize cells and their tissue environment, to understand how this environment drives cell behavior in health and disease. We worked for years to understand axon geometry and stiffness change in neurodegenerative diseases, and how this affects myelin repair. This knowledge of the interactions between neural cells and their environment allowed us to create artificial axons that mimic the key features of the brain environment that are important for biology of neural cells and myelination.
To mimic axons, we developed a novel biocompatible, ultraviolet-curable hydrogel. This material enabled the creation of very thin, freestanding fibers with extremely low stiffness, similar to real axons. To develop a reliable fabrication technology for our platform, graduate student Daniela Espinosa-Hoyos PhD ’20 and I then teamed up with the group of Professor Nicholas Fang in the Department of Mechanical Engineering, the experts in 3D printing. They built specialized 3D printers based on a technique called projection micro-stereolithography. Together, we developed a method that can reproducibly produce these complex micrometer-scale structures. This work built on an earlier collaboration with the group of Jennifer Lewis, a Harvard University professor, using direct inkjet printing of supported hydrogel fibers.