New findings released by MIT researchers could help energy companies implement a long-recognized process for converting heavy, high-sulfur crude oil into high-value, cleaner fuels such as gasoline without using hydrogen — a change that would reduce costs, energy use, and carbon dioxide emissions. The process involves combining oil with water under such high pressures and temperatures that they mix together, molecule by molecule, and chemically react. The researchers have produced the first detailed picture of the reactions that occur and the role played by the water in breaking apart the heavy oil compounds and shifting the sulfur into easily removable gases. They have also formulated models that show how best to mix the oil and water to promote the desired reactions — critical guidance for the design of commercial-scale reactors.
More than a third of the world’s energy needs are met using oil, and our reliance on that convenient, high-energy-density resource will likely continue for decades to come, especially in the transportation sector. But converting crude oil into lightweight, clean-burning, high-quality fuels such as gasoline, diesel, and jet fuel is getting harder. In the past, oil found in the ground tended to be lightweight, clean, and easily made into high-value fuels. Now, more and more of it is heavy, thick, tarry material that, when refined, yields a higher fraction of lower-value products such as asphalt along with solid chunks of waste called coke. Moreover, newly discovered oil contains ever-higher concentrations of sulfur, a contaminant that, when burned, produces gases that are now strictly regulated because they interfere with pollution-control systems in vehicles and contribute to acid rain and smog.
Processes now used to upgrade and desulfurize heavy crude oil are expensive and energy-intensive, and they require hydrogen, which companies typically produce from natural gas — a high-cost process that consumes valuable gas resources and releases high levels of carbon dioxide (CO2). “So there’s a lot of interest in finding alternative processes for converting low-quality crude oil into valuable fuels with less residual coke and for removing the sulfur efficiently and economically without using hydrogen,” says Ahmed Ghoniem, the Ronald C. Crane ('72) Professor of Mechanical Engineering at MIT.
One approach calls for using water rather than natural gas as the source of the hydrogen molecules needed for key chemical reactions in the refining process. Ordinarily, oil and water won’t mix, so the molecules can’t “see” one another and chemically react. But using “supercritical” water solves that problem. Under extreme conditions — specifically, at pressures and temperatures above 220 atm and 375 degrees Celsius — water goes into a supercritical state in which it is as dense as a liquid but spreads out to fill a confined space as a gas does. Add oil to supercritical water (SCW) and stir, and the two will mix together perfectly, setting the stage for the desired chemical reactions — without any added hydrogen from natural gas.
Industrial and academic researchers have demonstrated that mixing heavy oils with SCW produces lighter hydrocarbons (compounds of hydrogen and carbon atoms) containing less sulfur and forming less waste coke. But no one has understood exactly how it happens or how to optimize the process. For the past five years, Ghoniem and William Green, the Hoyt C. Hottel Professor of Chemical Engineering at MIT, have been working to close gaps in the fundamental knowledge about the chemistry involved as SCW and oil molecules react and about the flows and mixing behaviors that will produce the desired reactions and reaction products. Combining those new insights, the researchers are developing new computational tools to help guide energy companies that want to implement the new process. “Testing designs and operating conditions at large scale and extreme pressures is almost impossible,” Ghoniem says. “Our goal is to provide computer models that companies can use to predict performance before they start building new equipment.”
Tracking the chemistry
When crude oil is mixed with SCW, the hundreds of chemical compounds present can react together in different combinations and at different rates, in some cases producing intermediate compounds that are then involved in further reactions. “The challenge with SCW processing is that you have to let the oil and SCW mix together long enough for the reactions that remove sulfur and break down large hydrocarbon molecules to happen — and then stop the process to prevent further reactions that form products you don’t want,” Green says.
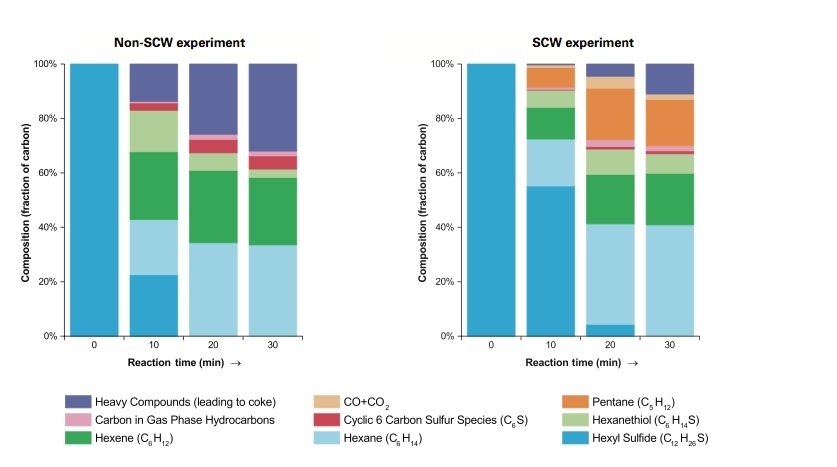
Based on a series of experiments, Green and his team have defined key chemical reactions that take place, how quickly they occur, and the intermediate products that are formed. As a sample sulfur-containing compound, they used hexyl sulfide, a large molecule made up of 12 carbon atoms, 26 hydrogen atoms, and one atom of sulfur. To test the impact of the SCW, they performed two parallel experiments. In one, they heated hexyl sulfide without adding water; in the other, they mixed the hexyl sulfide with SCW. In both cases, they removed samples from their reactor vessel at regular intervals up to 30 minutes.
Results are shown in Figure 1 in the slideshow above. As expected, both sets of samples include a variety of smaller hydrocarbon compounds, some with bound sulfur. But the SCW samples (at right) brought some surprises. The products include pentane — a smaller hydrocarbon not seen in the absence of SCW — and carbon monoxide and CO2. Since water is the only source of oxygen in the mixture, it must be reacting with the carbon compounds. In addition, in the SCW experiments, less of the carbon goes into heavy compounds that are likely to lead to coke formation. The appearance of the samples supports that conclusion: The non-SCW product is dark brownish-black in color, while the SCW product is a light yellow, clear liquid consistent with coke suppression by the SCW.
To further clarify the chemical reactions and how they are affected by temperature, pressure, and SCW concentration, the researchers combined their experimental work with theoretical modeling and analysis. Based on those studies, they identified the whole series of chemical reactions by which hexyl sulfide breaks down and releases its sulfur in the presence of SCW. According to that “reaction mechanism,” the sulfur-bearing hexyl sulfide is first broken apart, forming a smaller molecule with the sulfur atom in a very reactive form. In the absence of water, that highly reactive sulfur-bearing molecule would join with others like itself to form a long chain and eventually become coke. But in the presence of water, it reacts with the water, and the products ultimately include lighter hydrocarbons that are readily converted into valuable light fuels. The sulfur combines with hydrogen atoms to form hydrogen sulfide, a gas that can easily be removed and dealt with using existing technology.
Green notes that some of those reactions came as a surprise. “People didn’t expect them,” he says. “But we were able to discover that they were occurring and how fast they proceed and how much energy is needed to start them.” By knowing those “energy barriers,” the researchers can determine the reaction rates under different operating conditions — critical information for the overall model of the process.
Those results define — for the first time — the key roles played by water in the SCW system. “We confirmed that the hydrogen atoms needed to convert the sulfur to hydrogen sulfide can be provided by water rather than by hydrogen gas, as in the conventional process,” Green says. “And our empirical data show that the new SCW method does make less coke than the conventional process, for reasons that we’re now trying to clarify.”
Mixing without stirring
The results described thus far elucidate reactions and reaction rates under different conditions. Knowing what those conditions are inside a practical reactor is a parallel challenge. When oil is injected into flowing SCW, interactions between the two flows determine how mixing and heating proceed, first at the macroscale and then down to the microscale at which chemical reactions occur. The trick is to encourage and control optimal mixing. Using a stirring device is impractical, given the extreme supercritical conditions. So the researchers must generate such mixing naturally. “We need to know what drives the speed and patterns of mixing so we can select equipment designs and operating conditions that will either support key features of mixing for a long time or let them decay faster,” says Ghoniem.
To understand the details of flows and mixing, the researchers are using three-dimensional computational fluid dynamics (CFD), a method of simulating fluid flows within a well-defined region. Such modeling involves equations that describe the flow, mixing, and energy transfer between streams of fluids. But with supercritical fluids, key parameters such as viscosity and density are in ranges not seen under normal (non-supercritical) conditions. Nevertheless, the researchers were able to use powerful computers to accurately solve their CFD model, accounting for the complex changes that occur as fluids move from normal to supercritical conditions. To their surprise, they found that supercritical flows do indeed behave differently. For example, they become turbulent earlier than do comparable flows under normal conditions.
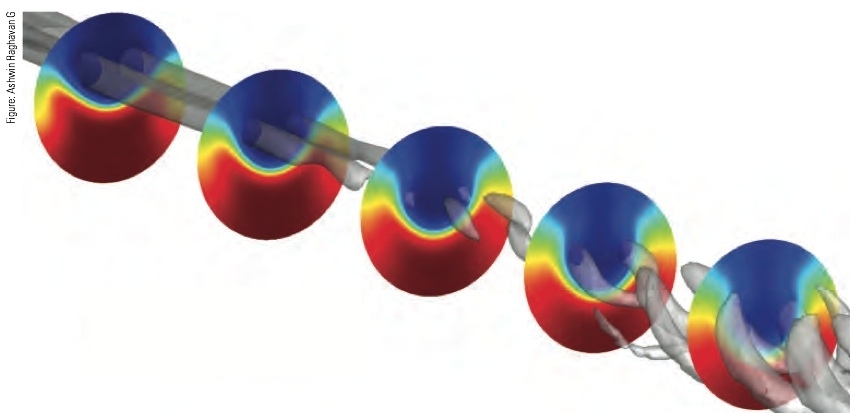
In one practical implementation of their model, they simulated mixing between SCW and oil near a “tee” junction consisting of a horizontal pipe with a smaller pipe coming into it from the top. SCW flows through the horizontal pipe, and cold oil — here a sample hydrocarbon — is injected into it through a vertical pipe. Figure 2 in the slideshow above shows how the SCW and oil mix as they flow down the pipe from left to right. (The walls of the circular pipe are not shown.) Initial interaction between the two streams causes the formation of two coherent swirls called vortices — rotating structures in the fluids shown in the figure as gray tubes. At first, the vortices are separate swirls that spin in opposite directions, mixing the oil and SCW together. Moving along the pipe, the vortices break down, and mixing rates decay.
The colored circles in Figure 2 show mixing between the two fluids at five cross sections located along the pipe. Blue regions are rich in cold oil; red regions are rich in hot SCW; and regions shown in intermediate colors have varying concentrations of the two fluids. The oil enters the cross section at the top and water at the bottom. As the spinning vortices form, the oil is driven downward near the center of the pipe, and the water is driven upward along the walls. In the first cross section, the interface layer between the oil and SWC is thin and sharp. In subsequent cross sections, that layer expands and diffuses, showing the extent of the mixing.
The researchers conclude that most of the fluid mixing and associated heat transfer is due to the swirling action of the vortices. However, they note that the mixing rate and heating rate differ — and that both influence the chemistry that occurs in regions where the fluids are mixed. Given design and operating details — the kind of oil; pressures, speeds, and temperatures of the incoming flows; shape and size of the pipes; and so on — the CFD simulation can predict “how this natural mixing process will progress and how temperatures will change at different locations over time,” Ghoniem says.
The researchers are continuing to generate new knowledge that will help SCW processing become an economically viable commercial option. For example, they are clarifying the reactions whereby carbon-carbon bonds are broken in the heaviest fractions, including asphalt. They are quantifying the different rates at which various oils will diffuse and mix in SCW — an effect first discovered in their modeling analyses. They are taking a closer look at inexpensive catalysts that can help encourage the breakdown of large hydrocarbons and are stable enough to be regenerated and reused. And they are exploring the possibility of linking SCW processing with other environmentally friendly desulfurization and upgrading technologies to create a combined system that will make it practical to continue producing high-value fuels from all kinds of oil for decades to come.
This research was supported by Saudi Aramco, a founding member of the MIT Energy Initiative.