There is no cure for amyotrophic lateral sclerosis (ALS), a disease that gradually kills off the motor neurons that control muscles and is diagnosed in nearly 6,000 people per year in the United States.
In an advance that could help scientists develop and test new drugs, MIT engineers have designed a microfluidic chip in which they produced the first 3-D human tissue model of the interface between motor neurons and muscle fibers. The researchers used cells from either healthy subjects or ALS patients to generate the neurons in the model, allowing them to test the effectiveness of potential drugs.
“We found striking differences between the healthy cells and the ALS cells, and we’ve been able to show the effects of two drugs that are in clinical trials right now,” says Roger Kamm, the Cecil and Ida Green Distinguished Professor of Mechanical and Biological Engineering at MIT and the senior author of the study.
MIT postdoc Tatsuya Osaki is the lead author of the paper, which appears in the Oct. 10 issue of Science Advances. Sebastien Uzel, a former MIT graduate student, is also an author of the paper.
3-D junctions
Scientists began developing tissue models of the connections between motor neurons and muscle cells, also called neuromuscular junctions, decades ago. However, these were limited to two-dimensional structures, which do not fully replicate the complex physiology of the tissue.
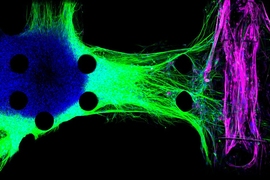
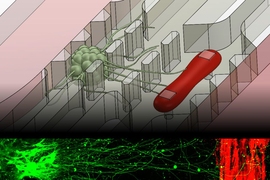
Kamm and his colleagues developed the first version of their 3-D neuromuscular junction model two years ago. The model consists of neurons and muscle fibers that occupy adjacent compartments of a microfluidic chip. Once placed in the compartments, the neurons extend long fibers called neurites, which eventually attach to the muscles, allowing the neurons to control their movement.
The neurons are engineered so that the researchers can control their activity with light, using a technique called optogenetics. The muscle fibers are wrapped around two flexible pillars, so when the neurons are activated by light, the researchers can measure how much the muscle fibers contract by measuring the displacement of the pillars.
In the 2016 version of the model, the researchers used mouse cells to grow the neurons and muscles, but differences between species can affect drug screening. In the new study, they used induced pluripotent stem cells from humans to generate both the muscle cells and the neurons. After demonstrating that the system worked, they began to incorporate neurons generated from induced pluripotent stem cells from a patient with sporadic ALS, which accounts for 90 percent of all cases.
This ALS model showed significant differences from the neuromuscular junctions created from healthy cells. The neurites grew more slowly and seemed to be unable to form strong connections with the muscle fibers, Kamm says.
“You can see that the healthy neurites are going directly to the individual myotubes and then activating them. However, the ALS neurons don’t seem to be able to connect very well,” he says.
This translated to weaker muscle control: After two weeks, the muscles innervated by ALS motor neurons were generating only about one-quarter the force produced by muscles controlled by healthy neurons. This also suggested that ALS motor neurons attacked healthy skeletal muscle tissues.
“The use of human-derived neuronal cells from ALS patients, combined with stem cell derived muscle cells — and the formation of a functional neuromuscular junction — is a major advance in the field of tissue models on a chip,” says Rashid Bashir, a professor of electrical and computer engineering and bioengineering at the University of Illinois at Urbana-Champaign, who was not involved in the research.
Promising drugs
The researchers then used their model to test two drugs that are now in clinical trials to treat ALS — rapamycin and bosutinib. They found that giving both of the drugs together restored most of the muscle strength that had been lost in the ALS motor units. The treatment also reduced the rate of cell death normally seen in the ALS motor unit.
Working with a local biotech company, Kamm and his colleagues hope to collect induced pluripotent stem cells from 1,000 ALS patients, allowing them to perform larger-scale drug studies. They also plan to scale up the technology so they can test more samples at a time, and to add more types of cells, such as Schwann cells and microglial cells, which play supportive roles in the nervous system.
This tissue model could also be used to study other muscular diseases such as spinal muscular atrophy, which affects nerve cells found in the spine.
The research was funded by the National Science Foundation through the Science and Technology Center on Emergent Behaviors of Integrated Cellular Systems.