The Zika virus circulated in many regions of the Americas for several months before cases of infection were detected, according to new data from an international research team from the Broad Institute of MIT and Harvard and several collaborating institutions.
These findings, revealed today in Nature in a paper led by Pardis Sabeti of the Broad Institute and Harvard University, arise from an analysis of 174 Zika virus genomes — including the largest collection of new Zika virus genomes to date — sequenced from patient and mosquito samples collected in 11 affected countries and territories.
The paper’s co-authors include Hayden Metsky, a graduate student in the Sabeti lab, MIT’s Department of Electrical Engineering, and the Computer Science and Artificial Intelligence Laboratory, who is one of the paper’s first authors; Lee Gehrke, the Hermann L.F. von Helmholtz Professor in MIT’s Institute for Medical Engineering and Science (IMES); and Irene Bosch, a research scientist at IMES.
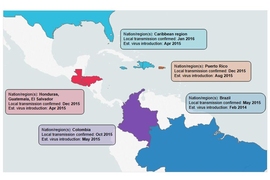
The genomic data allowed the research team to reconstruct for the first time the spread of the virus across South and Central America, the Caribbean, and into the southern United States.
In many of these regions, the virus circulated for months before local cases of infection were detected. Sabeti and colleagues’ analysis suggested that Zika was circulating in Brazil around February 2014, a year before that nation’s first confirmed infections were reported. Similarly, the virus appears to have arrived in Colombia, Honduras, Puerto Rico, and elsewhere in the Caribbean from 4.5 to 9 months before the first confirmed local infections, highlighting the importance of having sensitive and specific diagnostic tools early in an outbreak.
These results appear only now, months after the peak of the outbreak, because sequencing Zika virus has proved to be challenging, particularly when done directly from patient samples. The difficulty arises because Zika virus is typically present at very low levels in patients and disappears quickly. As a result, very few Zika genomes had been generated prior to this study, leaving researchers with little basis for understanding how the virus is spreading and evolving.
To address this lack of data, the team developed new laboratory and analytical methods for capturing robust Zika genomic data, and applied them to samples collected in partnership with collaborators in Brazil, Colombia, the Dominican Republic, Honduras, Jamaica, Puerto Rico, Massachusetts, and Florida to generate 110 new genomes for this study. The team combined those genomes with an additional 64 available in GenBank and in one of the study’s two companion papers, to carry out their analysis.
“We knew it was important to understand the viral populations driving the epidemic, which motivated us to tackle the challenges of sequencing Zika,” says Metsky. “Because the data we generated capture the geographic diversity of the virus across the Americas, they provide an opportunity to trace how and when the virus spread. Our data and findings will also support development of more effective molecular diagnostic tests, as well as improved public health surveillance tools.”
The work also highlights the importance of quickly creating trusted partnerships between researchers and across institutions and regions, and of sharing data openly during outbreaks.
“This collaboration has been about each partner sharing their unique resources and expertise — samples, protocols, analyses, insights — to help understand and fight Zika,” says Thiago Moreno L. Souza, a study co-senior author and senior research scientist at Fundação Oswaldo Cruz in Rio de Janiero, Brazil. “Sharing the data widely for the same end goal was an obvious extension of that ethos.”
The study was published together with two companion papers, one by Kristian Andersen from the Scripps Research Institute and colleagues examining Zika’s introduction into Florida, and the other by Oliver Pybus at Oxford University and colleagues examining the virus’s establishment and early spread within and beyond northeastern Brazil. All three teams committed to sharing data and ideas freely amongst themselves and to releasing their findings cooperatively and quickly.
“Collectively our goal was to capture as complete a picture of the genetic underpinnings of the epidemic in the Americas as we could. Working together was critical to reaching that goal,” says study co-senior author Bronwyn MacInnis, associate director of malaria and viral genomics in the Broad’s Infectious Disease and Microbiome Program. “Instead of competing for publication, we wanted our papers to leverage each other and reflect our commitment to the greater good.”
Zika remains a significant public health threat in affected countries and regions, highlighting the need for continued surveillance and research on the virus. According to MacInnis, the epidemic holds lessons about the role genomics can play in identifying and tracking emerging outbreaks early, before widespread infection occurs.
“Genomics allowed us to reconstruct how the virus traveled and changed across the epidemic — which also means that genomics could have helped detect it much earlier,” she says. “We were way behind the curve on Zika. We need to be well ahead of the next emerging viral threat, and genomics can have a role in achieving this.”
Support for this study was provided by Marc and Lynne Benioff, the National Institute of Allergy and Infectious Diseases, and other sources. Pardis Sabeti is an Investigator with the Howard Hughes Medical Institute.
The work was and continues to be part of a collaboration across nations. Those currently involved include the following investigators, together with their teams and collaborators: Thiago Moreno L. Souza, Patrícia T. Bozza, Wim Degrave, et al. at Oswaldo Cruz Foundation in Brazil; Fernando Bozza at Oswaldo Cruz Foundation and D’or Institute in Brazil; Salim Mattar at the University of Córdoba in Colombia; Luis A. Villar Centeno at the Industrial University of Santander in Colombia; Ivette Lorenzana at the National Autonomous University of Honduras in Honduras; Joshua Anzinger at The University of the West Indies in Jamaica; Andrew Rambaut at the University of Edinburgh in the UK; Sharon Isern and Scott F. Michael at Florida Gulf Coast University; Sandra Smole at the Massachusetts Department of Health; Irene Bosch and Lee Gehrke at MIT; and Kristian Andersen at The Scripps Research Institute.